Have you ever wondered what makes construction machinery so durable? The secret lies in the remarkable 35MnB steel. This article unveils how elements like Carbon, Silicon, Manganese, Boron, and Chromium transform this steel into a powerhouse of strength and resilience. Dive in to uncover the science behind its exceptional performance in demanding environments.

35MnB steel, a boron-alloyed medium-carbon manganese steel, is extensively employed in the fabrication of crawler chassis components for construction machinery. This material’s popularity stems from its exceptional hardenability and superior response to heat treatment processes, resulting in components with enhanced wear resistance and mechanical properties.
Key applications of 35MnB steel in crawler systems include:
The utilization of 35MnB steel in these applications ensures extended service life, improved performance under high-stress conditions, and reduced maintenance requirements for construction machinery operating in demanding environments.

Given the harsh operating conditions of its applications, the use of 35MnB steel requires it to be in a quenched and tempered state.
Hardenability and hardenability are crucial indicators for quenched and tempered steel, and their values are typically maintained by strictly controlling key elements such as Carbon (C), Silicon (Si), Manganese (Mn), Boron (B), and Chromium (Cr), which have significant impacts on hardenability.
The carbon content in the 35MnB steel determines the hardness achievable after quenching. A higher carbon content leads to higher quenching hardness, but also increases the risk of cracking and reduces the steel’s plasticity and impact toughness.
For crucial components such as the crawler chassis, to minimize the effect of carbon content fluctuation on surface hardness and quenching layer depth, it is necessary to establish requirements for selecting the carbon content. Generally, the upper and lower limits of carbon content are controlled within a range of 0.05%.
In addition to enhancing strength and hardenability, silicon in 35MnB steel also helps to eliminate gas from the steel and stabilize it during steelmaking.
However, as the silicon content increases, the plasticity and toughness of the steel decrease, and it becomes prone to forming a banded structure.
Manganese (Mn), which is the primary alloying element of 35MnB steel, improves the steel’s hardenability and lowers its critical cooling rate. Mn forms a solid solution with ferrite during heating, enhancing the strength of the steel. Mn is typically used when the depth of the hardened layer is greater than 4mm. This is because it reduces the critical cooling rate, resulting in more uniform quenching hardness even when the cooling conditions are not stable.
As shown in Fig. 1 and 2, when the Mn content in the steel is 1.10%, it greatly improves the strength of the steel with only a minor decrease in plasticity and a slight improvement in toughness. However, if the Mn content exceeds this amount, the hardenability and strength will continue to improve, but the toughness will drop significantly.
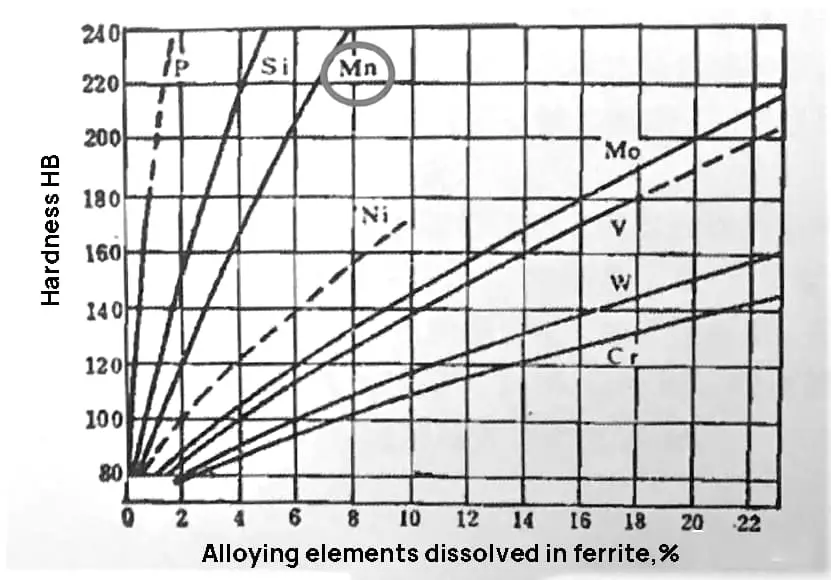
Fig. 1 Effect of alloying elements on solid solution strengthening
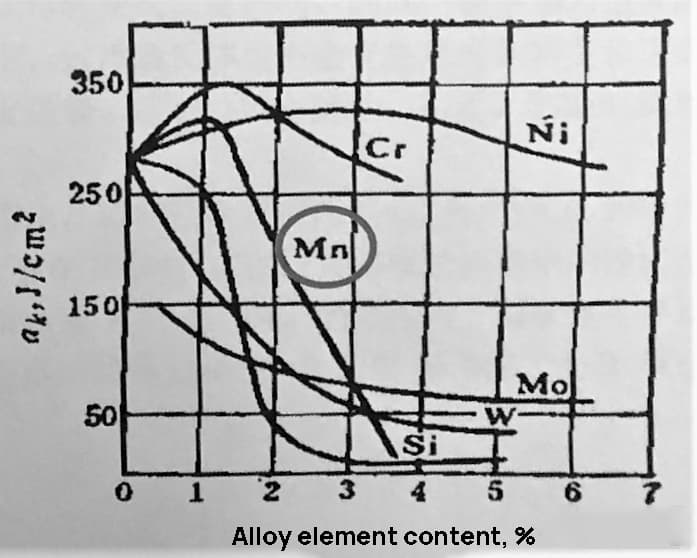
Fig. 2 Effect of alloying elements on impact energy of ferrite
In quenched and tempered high-strength steel, the addition of alloy element B can enhance hardenability. This is achieved by dissolving a small amount of B in high-temperature austenite. During cooling, B will segregate at the austenite grain boundaries, inhibiting ferrite nucleation and thus improving hardenability, especially at low cooling speeds.
However, B in steel is an active element that easily reacts with N to form stable BN, which is insoluble at quenching temperatures. This reduces the amount of effective B in the austenite solid solution and reduces its hardenability-enhancing effects.
To improve hardenability, it is necessary to add nitride-forming elements, control N elements, and maintain the solid solution amount of B in austenite. Additionally, when the B content exceeds 30ppm, the material’s plasticity and toughness will significantly decrease.
Elements such as V, Ti, Al, and B are strong nitride-forming elements in steel and form the nitrides VN, AlN, BN, and TiN, respectively. When added to steel containing B, N in the steel will preferentially precipitate as TiN or Ti (C, N). This precipitation starts at temperatures higher than 1400℃, much higher than the precipitation start temperature of BN. As the temperature decreases, the proportion of solid N in TiN increases, fixing N in the steel and preventing BN formation, thus increasing the effective B content in austenite and improving hardenability.
To maximize the effective B content, it is important to control the TiN ratio in steel, with an ideal value of 3.42. If the ratio is less than 3.42, residual N content will increase and BN precipitation will occur, reducing the effective B content, hardenability, and increasing brittleness. To avoid these effects, it is important to strictly control residual N content in steel.
Cr is an element that greatly enhances the hardenability of steel.
The addition of Cr to medium carbon chromium steel increases the phase transformation incubation period, causing the isothermal transformation curve to shift to the right. This also causes pearlite transformation to occur at higher temperatures and bainite transformation to occur at lower temperatures.
As a result, when the right amount of chromium is added to steel, even with slow cooling during the quenching process, undercooled austenite will not transform into pearlite or bainite before reaching the martensite transformation temperature, significantly improving the steel’s hardenability.
However, Cr also significantly exacerbates the temper brittleness of nickel and manganese steels. Therefore, the Cr content in 35MnB steel is carefully regulated.
Studies on the effect of trace Cr on the hardenability of 35MnB crawler link steel indicate that even small changes in Cr content (Cr ≤ 0.20%) can significantly impact hardenability, especially when Cr content exceeds 0.10%. This significantly improves the steel’s hardness, particularly at points far from the water-cooled end.
The figure below shows that the quenching hardness can increase by 2 to 3 HRC on average within the range of 1.5 to 20.0 m from the water-cooled end. When the distance from the water-cooled end is more than 20.0 m, the hardness increases even further, by about 6 HRC.
Additionally, the diameter of the quenchable round bar of 35Mnb steel containing Cr0.18% is about 20mm larger than that of steel containing Cr0.02%.
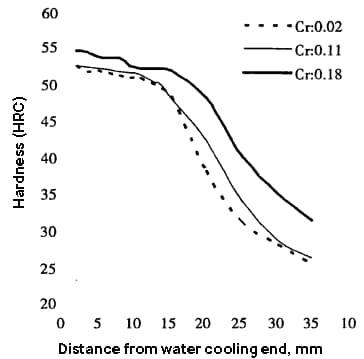
Fig. 3 Effect of Cr content on Hardenability
Because Cr has the ability to form carbides, it requires an increase in heating temperature and an extended heating time, which is not ideal for induction hardening.
During the steel-making process, the high melting point of tin causes it to precipitate in the liquid phase before casting and solidification. This results in the formation of tin particles in the liquid, which are typically 2-10 μm in size.
These particles have a square, rhombus, or triangular shape (different from BN, as shown in Fig. 6), and exhibit an extremely high hardness (greater than 1000V).
As demonstrated in Fig. 4 and Fig. 5, these particles cannot be altered through any processing method and cannot be dissolved through high-temperature solid solution. Additionally, they lead to a large impact energy dispersion.
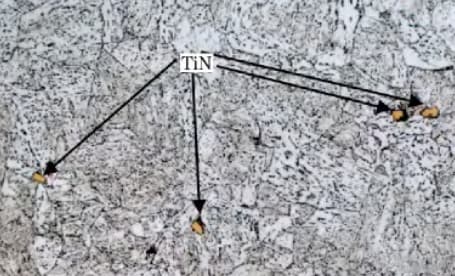
Fig. 4 observation under tin light microscope
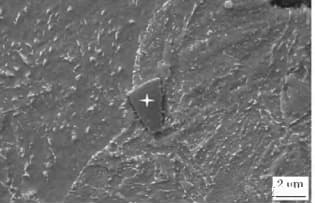
Fig. 5 observation of tin under electron microscope
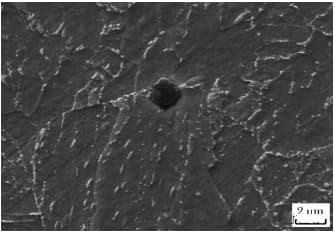
Fig. 6 observation of BN under electron microscope
Fig. 7 is a solubility product curve in liquid iron at 1400 ℃, 1450 ℃ and 1500 ℃;
As shown in the figure, when the temperature of the molten steel at the start of solidification is 1500℃, the presence of 80ppm of N and more than 0.043% of Ti in the steel will result in liquid and tin precipitation. Similarly, when the N content in the steel is 40ppm and the Ti content exceeds 0.086%, there will be liquid and tin precipitation.
When the final solidification temperature of the dendrite molten steel is 1400℃, the presence of 80ppm of N and more than 0.012% of Ti will result in liquid and tin precipitation. Additionally, if the N content in the steel is 40ppm and the Ti content exceeds 0.024%, there will be liquid and tin precipitation.
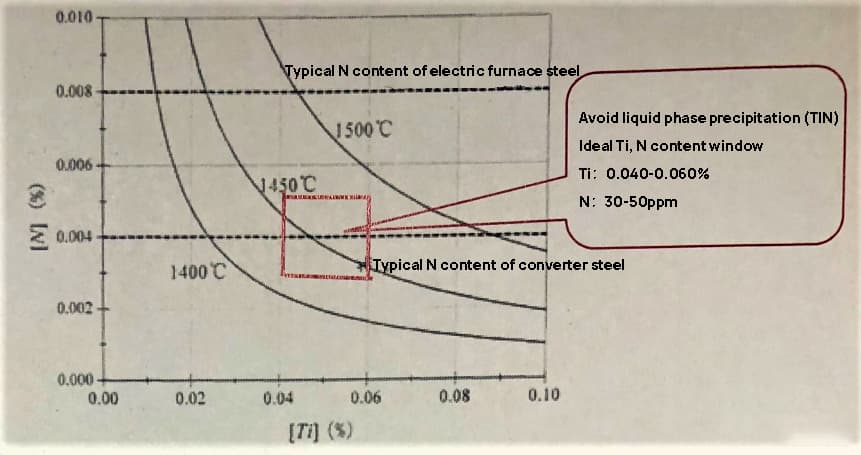
Fig. 7 tin solubility product curve
To prevent the occurrence of liquidus tin, it is crucial to adjust the content of Ti and N in steel appropriately. This will suppress the precipitation of liquidus tin during solidification and increase the cooling speed during casting to reduce the precipitation in the last solidified molten steel. By accelerating the cooling speed, there won’t be enough time for precipitation to occur dynamically.
Calculations of the solubility product of tin in liquid iron show that the final solidification temperature during smelting and pouring is approximately 1495°C, with an equilibrium solubility product of tin of 0.00302.
If the N content is controlled at 80 ppm, the maximum amount of tin that can be dissolved in liquid iron at the final solidification temperature is 0.0413%. To avoid liquid precipitation of tin, the chemical composition should have a Ti content of ≤ 0.0413%.
If the nitrogen content is controlled at 60 ppm, the maximum T content that can be dissolved in liquid iron at the final solidification temperature is 0.05%. To avoid producing liquidus tin, the design Ti content of the steel chemical composition should be ≤ 0.05%.
To increase the effective B content in 35MnB steel, the N content in the steel should be reduced to below 60 ppm.
If the liquid phase precipitation of tin exceeds 6 μm, it can greatly reduce the material’s fatigue life and impact toughness. If it exceeds 6 μm, it should be judged as Al2O3 brittle inclusion.
Inclusions such as tin, Al2O3, MgO · Al2O3, and Cao · Al2O3, which are hard and brittle, do not have plasticity under deformation temperature. They are easily separated from the body structure during deformation, damaging its continuity. In severe cases, cracks or cavities can appear at the edge of the undeformed inclusion.
In service, alternating stress can easily cause stress concentration, becoming a source of metal fatigue.
Good material composition control is essential to guarantee material performance. The recommended composition (in weight percentage) for 35MnB material during melting is as follows:
| Grade | 35MnB |
| C | 0.32-0.36 |
| Si | 0.15-0.35 |
| Mn | 1.1-1.4 |
| P | ≤0.025 |
| S | 0.025 |
| Cr | 0.15-0.25 |
| Ni | 0.2 |
| Cu | 0.25 |
| B | 0.0005-0.003 |
| Al | 0.015-0.045 |
| Ti | ≤0.05 |
| Mo | ≤0.05 |
| 【H】 | ≤2ppm |
| 【O】 | ≤18ppm |
| 【N】 | ≤60ppm |