Have you noticed odd white spots on your 6063 aluminum alloy products? These surface defects, often appearing after sulfuric acid anodizing, can compromise the quality of your metalwork. This article delves into the reasons behind these oxidation defects, including issues with alkali etching, ingot quality, extrusion-heat treatment, and the anodizing process. By understanding these causes, you’ll learn effective solutions to mitigate these blemishes, ensuring your aluminum products maintain their desired appearance and performance. Discover how to prevent these common yet troubling defects in your aluminum alloys.

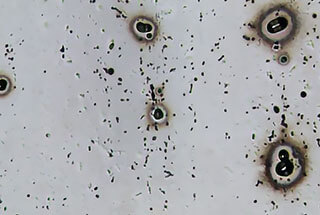
In actual production, T5 state 6063 aluminum alloy extrusion profiles with high processing rates (ε>95%) and thin walls (δ≤1.5mm) exhibit regular (and sometimes irregular) distribution of white spots (or non-luminous marks) on their surfaces after sulfuric acid anodizing.
In severe cases, dark blemishes—”white spots”—appear.
The distribution and characteristics of “white spots” are as follows: they are a type of surface defect that appears at roughly equal intervals, in line or flattened quadrilateral or irregular star point (flake) shapes, on planes parallel to the extrusion direction, with minor depth forming a groove relative to the base surface.

White spots are usually distributed on one or several surfaces of the profile, and sometimes they are distributed on all surfaces (for thin-walled hollow profiles, they are distributed on both sides of a certain plane or curved surface).
It was observed on-site that “white spots” form during the “alkali etching” process, and do not disappear after subsequent dilute nitric acid (or sulfuric acid) “neutralization”. After sulfuric acid anodizing, they are even more clearly presented.
The author specially cut two “white spot” samples with larger areas (F=30-40mm2) from the alkali etched wash (the solution contains ω(Zn2+)≥5×106). Then, a DV-5 atomic emission spark direct-reading spectrometer was used to quantitatively analyze the components of the “white spot” areas of the two samples. The results are as follows (all data in the table are mass fractions):
From the analysis results of Table 1, it can be seen that the contents of Si, Mg, and Zn elements in the “white spots” have significantly increased. However, the results of Table 2 show that the contents of Si and Zn elements in the “white spots” have significantly increased, while the content of Mg elements has decreased.
From the perspective of metal material corrosion, this surface defect of Mg2Si is essentially the result of “exfoliation corrosion” of the 6063 aluminum alloy material.
Exfoliation corrosion is a type of superficial selective corrosion where corrosion takes place along the metal surface, and the volume of its products is often much larger than the corroded metal, thereby expanding.
Generally speaking, when aluminum is adjacent to dissimilar metals with cathodic properties, “exfoliation corrosion” escalates. Observations under an electron microscope found that “exfoliation corrosion” usually proceeds along insoluble constituents (such as Si, Mg2Si, etc.) or along grain boundaries.
The primary phase composition of 6063 aluminum alloy includes the α(Al) solid solution, free Si(anode phase), and FeAl3(anode phase). When the iron content is high, the β(FeSiAl)(anode phase) is present; when the iron content is low, the α(FeSiAl)(cathode phase) is present. Other possible impurity phases include MgZn2, CuAl2, etc.
During production, the 6063 aluminum alloy ingot often presents macro-segregation or intra-crystalline segregation due to the non-equilibrium crystallization process. Consequently, elements such as Si, Mg, Zn, and Cu distribute unevenly within the ingot.
Some aluminum profile processing enterprises, often for economic reasons, rarely perform homogenizing annealing treatment on small-sized ingots (e.g., less than φ100mm) to eliminate segregation phenomenon, thus paving the way for the creation of “white spots.
To improve production efficiency, low-temperature high-speed extrusion is commonly adopted in production operations. The “thermal effect” caused by the extrusion speed significantly increases the quenching temperature of the product at the die exit.
Upon contact with a graphite board (or wheel) with a surface temperature of 80-110 (or slightly lower) on a fixed output table, the profile surface undergoes a “rapid cooling heat exchange,” causing the concentration of alloy elements Mg and Si in that part to be higher than that in normal areas.
In the subsequent artificial aging process, this area will precipitate coarse β ′(Mg2Si) phase. 6063 aluminum alloy ingots, which haven’t undergone homogenizing annealing treatment and have a low heating temperature, due to the insufficient “thermal effect” caused by extrusion, cannot raise the quenching temperature of the profile to above 500.
This not only results in a small portion of the β(Mg2Si) phase in the ingot remaining in the profile structure but also triggers the previously mentioned changes in the Mg and Si elements that are high-temperature solid solutions in the α(Al) matrix phase. These factors prepare the structural conditions for the appearance of “white spots.”
For Si content that is greater than Fe content, excess Si is prone to aggregate in the α(Al) crystal or near the crystal boundary, forming a free monocrystal Si phase. The cathode phase Si and the segregated anode phase Mg2Si, or the anode phase α(Al) matrix and the coarse cathode phase Mg2Si, induce a “primary battery effect” in the alkali corrosion solution.
The result is the rapid dissolution of the α(Al) solid solution around the free Si or the preferential dissolution of the coarse Mg2Si phase compared to the α(Al) solid solution, leaving shallow and flat “corrosion pits” on the profile surface.
In addition, some researchers suggest that the white spots are related to the hydrolysis reaction of NaAlO2. When the ratio of Al3+ concentration to the total NaOH concentration exceeds 0.35, the stability of NaAlO2 decreases, and the hydrolyzed Al(OH)3 precipitates on the aluminum material surface.
Incomplete water washing can also easily result in spot or blocky “white spots.” However, it is believed that this is mainly related to the effect of scale inhibitors (such as hydroxycarboxylates, sodium tartrate, etc.) contained in the alkali corrosion additives.
Specifically, under stable alkali corrosion process conditions, hydroxycarboxylates can reversibly complex with Al(OH)3 to form soluble complex anions.
Generally, when the concentration of sulfuric acid is too high, the electrolysis temperature is excessively elevated, or when the Al3+ content in the sulfuric acid solution of the oxidation tank exceeds 20g/L, the following ionization equilibrium condition at a normal temperature (around 20 degrees) is disrupted.
With the increase of Al3+, Al(OH)3 in the sulfuric acid oxidation tank precipitates and adheres to the profile surface grooves or within the Al2O3 film pinholes in a flocculated form. It cannot be thoroughly washed with clean water, nor is it easy to seal the pores. Upon air drying, white spots appear on the surface.
① Strictly control the chemical composition, requiring that the excess of Si not exceed 0.20% and the Zn content not exceed 0.05%. Additionally, strive to uniformly anneal the ingots, and apply rapid cooling to the ingots after treatment.
② Modify the axis of the first graphite roll on the fixed discharge table to make its height adjustable. If possible, use a material with better insulation than graphite.
③ Employ low limit temperature extrusion to avoid local overheating, or minimize the overheating duration, so that the 6063 aluminum alloy doesn’t have sufficient time to precipitate the β′(Mg2Si) phase.
④ Add to the caustic etching solution a precipitant, Na2S or sodium hydrosulfide, in a quantity equivalent to twice the mass necessary to form ZnS precipitate. When Al3+ in the alkali solution exceeds the control standard, timely supplement with caustic etching additives.