
What makes stainless steel so resilient and versatile? The secret lies in its alloying elements. In this article, we uncover how elements like chromium, nickel, and molybdenum enhance the corrosion resistance and mechanical properties of stainless steel. From kitchen appliances to aerospace engineering, the right mix of these elements ensures superior performance and durability. Join us to explore the science behind this indispensable material and understand how each component contributes to making stainless steel a powerhouse in various applications. Dive into the fascinating interplay of elements that define its excellence.


1. According to chemical composition, it can be divided into: chromium stainless steel, chromium nickel stainless steel, chromium manganese stainless steel, chromium nickel molybdenum stainless steel, ultra-low carbon stainless steel, high molybdenum stainless steel, high-purity stainless steel, etc.
2. According to the metallographic structure, it can be divided into: martensitic stainless steel, ferritic stainless steel, austenitic stainless steel, austenitic ferritic stainless steel, etc.
3. According to the performance characteristics and uses of steel: such as nitric acid resistant (nitric acid grade) stainless steel, sulfuric acid resistant stainless steel, pitting corrosion resistant stainless steel, stress resistant stainless steel, high-strength stainless steel, etc.
4. According to the functional characteristics of steel: such as low-temperature stainless steel, non-magnetic stainless steel, free cutting stainless steel, superplastic stainless steel, etc.
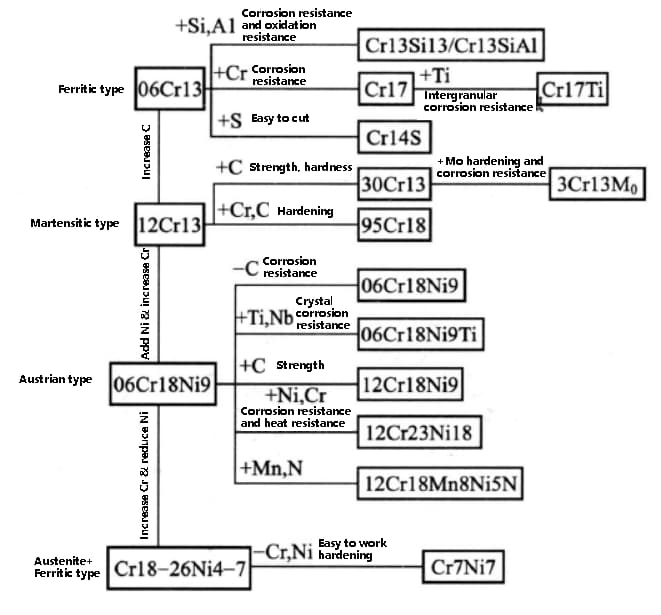
The development process of stainless steel grades is shown in the figure below:
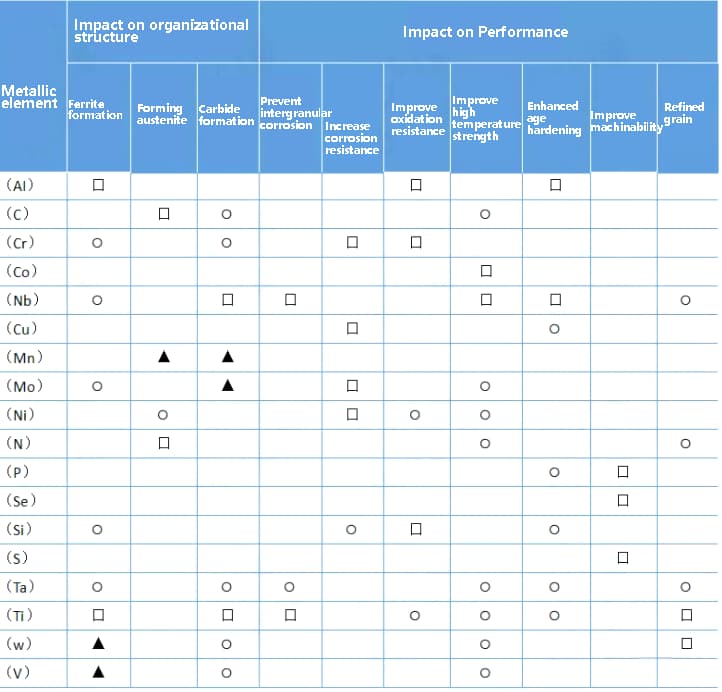
Note: 口 – strong effect, ⚪—— Moderate action, ▲ – weak action
(1) To achieve a stable anodic polarization curve of the passivation zone for a specific medium, ensure that the stainless steel is prepared appropriately.
(2) Increasing the electrode potential of the stainless steel substrate while reducing the electromotive force of the corrosive galvanic cell can help to improve its corrosion resistance.
(3) Enhancing the steel’s single-phase structure and reducing the number of micro batteries can improve its corrosion resistance.
(4) To form a stable protective film on the surface of steel, adding elements such as silicon, aluminum, and chromium can help create a dense protective film in many corrosion and oxidation situations, thereby enhancing the steel’s corrosion resistance.
(5) Eliminating or reducing various uneven phenomena in steel is also a vital step in enhancing its corrosion resistance.
Adding alloy elements to steel is the primary method used to improve its corrosion resistance.
Adding different alloy elements can work in one or several ways simultaneously to improve steel’s corrosion resistance.
The type and content of alloy elements have a direct impact on the corrosion resistance of stainless steel. The primary function of alloy elements is to influence the polarization performance of iron and electrode potential.
The anodic polarization process of commonly used metals such as Fe, Cr, Ni, and Ti follows a unique polarization pattern.
Following anode passage, the anode potential increases, and the anode current (corrosion rate) changes accordingly, almost with the same pattern.
The typical form of the polarization curve is shown in the figure below.
As the anodic polarization potential increases, the corrosion current does not decrease uniformly. Instead, it first increases, then decreases to a minimum, and maintains this current through a certain potential increase stage before increasing again.
This polarization curve is referred to as the anodic polarization curve with activation and passivation transition. It is divided into three regions: the activation region (A), passivation region (B), and overpassivation region (T).
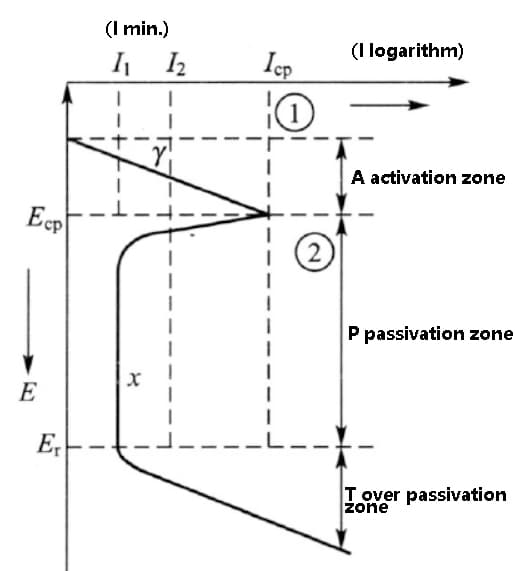
Fig. anodic polarization curve of activated and passivated transition metals
Polarization plays a significant role in improving the corrosion resistance of metals. Factors that enhance anodic or cathodic polarization can boost corrosion resistance, while factors of depolarization can reduce it.
Different alloy elements have varying effects on the polarization properties of iron. Elements that expand the passivation zone, which reduces the potential of the ECP and P zone and increases the potential of the Er point, can improve the corrosion resistance of steel. On the other hand, all elements that enhance passivation performance, causing the ICP and I1 points to move to the left, can reduce the corrosion current and improve the corrosion resistance.
Elements that increase the Er point potential tend to reduce pitting corrosion because, when the potential fluctuates near the over-passivation potential and the Er point potential is low, it can lead to local breakdown of the passivation film, resulting in pitting corrosion.
Among the alloy elements commonly used in steel, Cr can significantly improve the passivation performance of pure iron, increase the potential of Ecp, Ep, and Er points, and shift the position of Icp and I1 points to the left. Therefore, it is the most effective element in enhancing the corrosion resistance of iron.
In addition to Cr, alloy elements such as Ni, Si, Mo, etc. can also improve the passivation performance and expand the passivation zone to varying degrees.
Mo, for example, not only enhances the passivation performance of iron, but also increases the potential of the Er point, which improves the pitting corrosion resistance of iron.
In general, the electrode potential of a metal solid solution is lower than that of other compounds. Therefore, during the corrosion process, the metal solid solution is more likely to corrode as an anode.
One way to enhance the corrosion resistance of iron is to increase its electrode potential. Studies have demonstrated that adding Cr to iron to form a solid solution can significantly boost the electrode potential of the resulting material, as depicted in the figure below.
By elevating the electrode potential of a material, its corrosion resistance can be markedly enhanced.
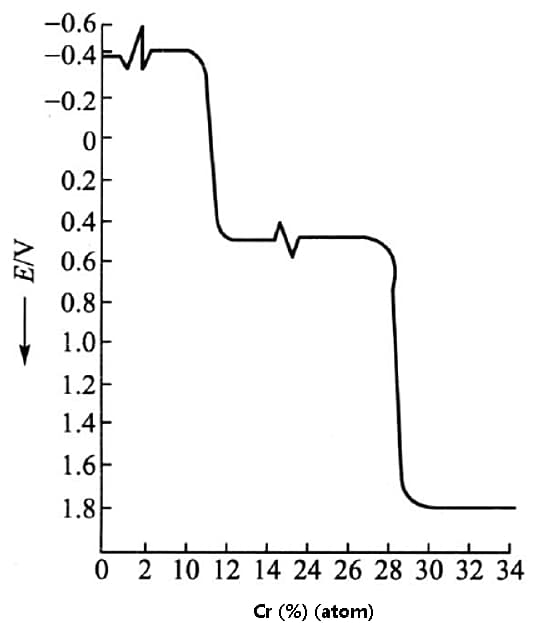
Fig. effect of chromium on electrode potential of Fe Cr alloy
Due to the good effect of chromium on iron passivation and electrode potential, chromium has become the main alloying element of various stainless steels.
The matrix structure of stainless steel is crucial for achieving the desired mechanical and process properties, as well as ensuring excellent corrosion resistance.
Two types of stainless steels, single-phase ferritic steel, and single-phase austenitic steel, exhibit superior corrosion resistance.
The effect of alloy elements on the matrix structure primarily depends on whether they act as ferrite (α) stabilizers or austenite (γ) stabilizers.
When the stabilizing element is dominant, single-phase α stainless steel can be obtained; otherwise, single-phase γ stainless steel is obtained.
1. Chromium
Chromium is the primary element that determines the corrosion resistance of stainless steel. When the chromium content (atomic ratio) reaches between 1/8 and 2/8, the electrode potential of iron jumps, leading to an improvement in the steel’s corrosion resistance. Chromium is also a stabilizing element that helps to enhance the material’s overall durability.
One reason for this is that chromium oxide is relatively dense and can form a protective film that resists corrosion.
2. Carbon and nitrogen
Carbon plays an essential role in stainless steel production, as it strongly stabilizes austenite, with a stabilizing ability about 30 times greater than nickel. Additionally, carbon is the main element used for strengthening stainless steel. However, carbon can also form a series of carbides with chromium, which can significantly impact the corrosion resistance of stainless steel. Furthermore, carbon can worsen the processing and welding properties of stainless steel and cause ferritic stainless steel to become brittle.
Therefore, it is crucial to carefully control and apply carbon during the production and development of stainless steel. The combination of carbon and chromium has a significant effect on the formation of stainless steel structures, as demonstrated in the figure below.
The figure shows that when the carbon content is low and the chromium content is high, a ferrite structure is obtained, whereas a martensite structure is obtained when the carbon content is high and the chromium content is low.
In chromium stainless steel, a carbon content increase will lead to the formation of martensite when the chromium content is below 17%. On the other hand, a low carbon content and 13% chromium content will result in the formation of ferritic stainless steel.
As the chromium content increases from 13% to 27%, the ability to stabilize ferrite increases, which, in turn, causes an increase in carbon content (from 0.05% to 0.2%). Despite the rise in carbon content, the ferrite matrix can still be maintained.
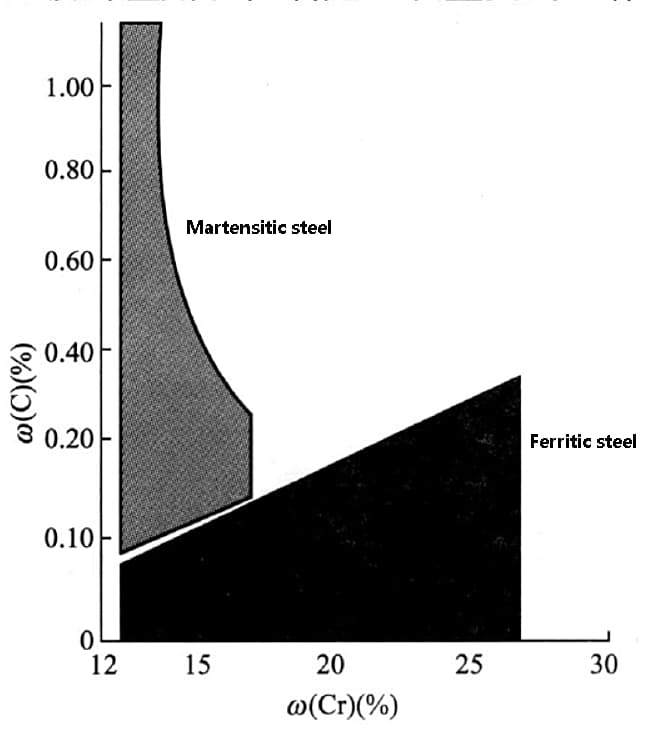
Fig. effect of carbon and chromium on Microstructure of stainless steel
3. Nickel
Nickel is one of the three important elements in stainless steel, as it can improve the corrosion resistance of the material. As a γ phase stabilizing element, nickel is the main component required to obtain single-phase austenite and promote its formation in stainless steel.
One of the key benefits of nickel is that it can effectively reduce the Ms point, keeping austenite stable at very low temperatures (-50 ℃) without undergoing martensitic transformation. However, increasing the nickel content will reduce the solubility of carbon and nitrogen in austenitic steel, thus increasing the tendency of these compounds to desolvate and precipitate.
As the nickel content increases, the critical carbon content of intergranular corrosion decreases, making the steel more susceptible to this type of corrosion. However, the effect of nickel on the pitting corrosion resistance and crevice corrosion resistance of austenitic stainless steel is not significant.
In addition to its corrosion resistance benefits, nickel can also improve the high-temperature oxidation resistance of austenitic stainless steel. This is mainly due to nickel’s ability to improve the composition, structure, and properties of chromium oxide film. However, it’s worth noting that the presence of nickel can reduce the high-temperature vulcanization resistance of steel.
4. Manganese
Manganese is a relatively weak austenite-forming element, but it plays a crucial role in stabilizing the austenite structure.
In austenitic stainless steel, manganese partially replaces nickel, and 2% Mn is equivalent to 1% Ni.
Manganese can also enhance the corrosion resistance of chromium stainless steel in organic acids, such as acetic acid, formic acid, and glycolic acid, and it is more effective than nickel.
However, when the chromium content in steel exceeds 14%, adding manganese alone cannot result in a single austenite structure.
Since austenitic stainless steel has better corrosion resistance when the chromium content is above 17%, the industry mainly employs Fe-Cr-Mn-Ni-N steel, such as 12Cr18Mn9Ni5N, as a replacement for nickel-containing alloys. The amount of nickel-free Fe-Cr-Mn-N austenitic stainless steel used is relatively lower.
5. Nitrogen
In the early stages, nitrogen was mainly used in Cr-Mn-N and Cr-Mn-Ni-N austenitic stainless steels to save Ni. However, in recent years, nitrogen has become an essential alloying element of Cr Ni austenitic stainless steel.
Adding nitrogen to austenitic stainless steel can stabilize the austenitic structure, improve the strength, and enhance the corrosion resistance, especially for local corrosion such as intergranular corrosion, pitting corrosion, and crevice corrosion.
In ordinary low-carbon and ultra-low-carbon austenitic stainless steel, intergranular corrosion resistance can be improved. Nitrogen affects the precipitation process of chromium carbide during sensitization treatment, increasing the chromium concentration at the grain boundary.
In high-purity austenitic stainless steel, where there is no precipitation of chromium carbide, nitrogen increases the stability of the passive film and reduces the average corrosion rate. Although chromium nitride precipitates in steel with high nitrogen content, the precipitation rate of chromium nitride is slow. Thus, sensitization treatment will not cause intergranular chromium deficiency, having little effect on intergranular corrosion.
Nitrogen can also inhibit the segregation of phosphorus at the grain boundary and improve the intergranular corrosion resistance of steel.
Currently, nitrogen-containing austenitic stainless steel mainly has high strength and corrosion resistance. It can be divided into three types: nitrogen control type, medium nitrogen type, and high nitrogen type.
The nitrogen control type involves adding 0.05%~0.10%N to ultra-low carbon (C ≤ 0.02%~0.03%) Cr Ni austenitic stainless steel to improve the strength, optimize the intergranular corrosion resistance, and enhance the stress corrosion resistance of the steel.
The medium nitrogen type contains 0.10%~0.50%N and is smelted and poured under normal atmospheric pressure. On the other hand, the nitrogen content of high nitrogen type is more than 0.40%.
It is generally smelted and poured under increasing pressure conditions. This type of steel is mainly used in the solid solution state or semi-cold working state, as it possesses high strength and corrosion resistance.
Currently, high nitrogen austenitic steel with a nitrogen content ranging from 0.8% to 1.0% has been successfully applied in practical applications and has started industrial production.
6. Titanium, niobium, molybdenum and rare earth elements
Titanium and niobium are elements that can strongly form carbides, which can preferentially react with carbon than chromium, thereby preventing intergranular corrosion and improving the steel’s corrosion resistance.
When adding titanium and niobium to the steel, it is important to maintain a certain proportion with the carbon content.
Molybdenum, on the other hand, can enhance the passivation ability of stainless steel and broaden the range of passivation media. This means it can withstand hot sulfuric acid, dilute hydrochloric acid, phosphoric acid, and organic acids. The passivation film created with molybdenum is highly stable in numerous media and is less likely to dissolve.
Stainless steel containing molybdenum is resistant to pitting corrosion since it can protect the passive film from damage caused by Cl-.
When rare earth elements like Ce, La, and Y are added to stainless steel, they can slightly dissolve in the matrix. This process helps purify the grain boundary, modify the inclusions, homogenize the structure, and reduce the precipitation of precipitates and segregation at the grain boundary. This leads to an improvement in the steel’s corrosion resistance and mechanical properties.
The influence of alloy elements on the matrix structure of stainless steel can be classified into two categories:
When these elements with different functions are added to steel simultaneously, the microstructure of stainless steel depends on their comprehensive effects.
To simplify the treatment, the effect of ferrite-forming elements is converted into the effect of chromium, known as chromium equivalent [Cr], while the effect of austenite-forming elements is converted into nickel equivalent [Ni].
Based on the chromium equivalent [Cr] and nickel equivalent [Ni], a diagram is created to represent the actual composition of the steel and the resulting structural state, as shown in the following figure.
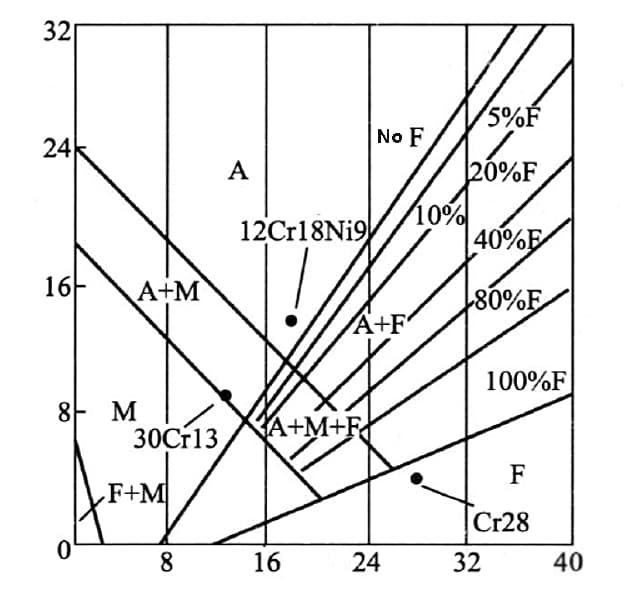
Fig. stainless steel structure diagram
The figure illustrates that 12Cr18Ni9 steel belongs to the austenitic stainless steel family and is located in the phase a zone.
On the other hand, Cr28 stainless steel is classified as ferritic stainless steel and can be found in the ferritic phase zone.
Meanwhile, 30Cr13 stainless steel falls under the category of martensitic stainless steel and is situated in the martensitic phase zone.
To achieve a single-phase austenite structure, a specific balance of alloy elements is necessary. Otherwise, some amount of ferrite structure will appear in the steel, resulting in a multiphase structure.
The strengthening of stainless steel is achieved through various mechanisms, including solid solution strengthening, phase transformation strengthening, second-phase strengthening, grain refinement strengthening, precipitation strengthening, and substructure strengthening.
The figure below illustrates the contribution of these mechanisms to the yield strength in 8%~10%Ni austenitic stainless steel.
As depicted in the figure, chromium, silicon, and carbon provide solid solution strengthening to the matrix, resulting in a several-fold increase in the yield stress of the austenitic matrix.
Another strengthening mechanism is the existence of α ferrite as the second phase, along with the refinement of grain size and precipitation of precipitates, which significantly enhances the strength of austenite.
The figure highlights that, in austenitic stainless steel, solid solution strengthening is a crucial mechanism, and grain refinement contributes the most to the overall strength.
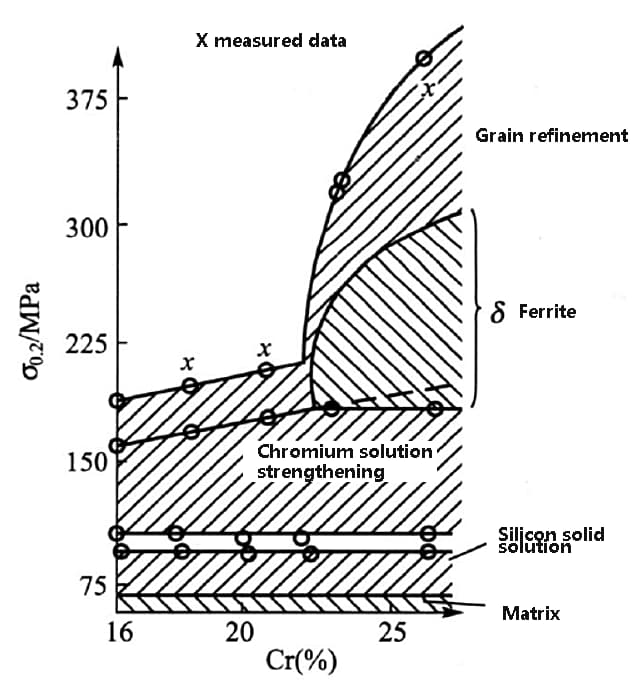
Fig. factors affecting the strength of austenitic stainless steel
The properties of different stainless steels vary depending on their composition and structure.
Please refer to the figure below for a comparison of the strength and plasticity of various stainless steels.
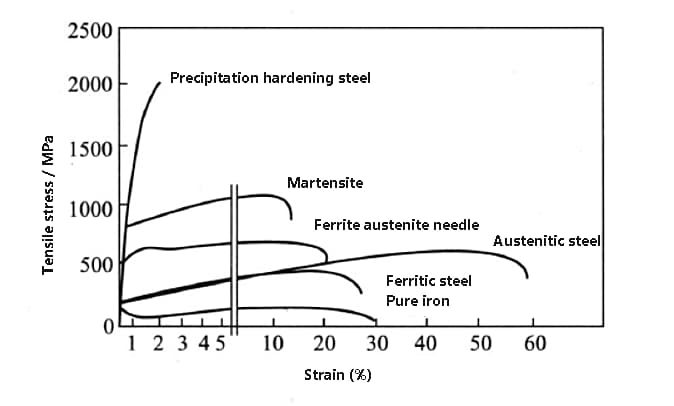
Fig. strength and plasticity comparison of various stainless steels and pure iron
Among all stainless steels, austenitic stainless steel has the best ductility, while precipitation-hardening stainless steel has the highest strength.
Martensitic stainless steel exhibits good overall mechanical properties, characterized by high strength and some degree of ductility.
Duplex stainless steel, which is a combination of ferritic and austenitic stainless steels, exhibits higher strength and better ductility.
Ferritic stainless steel and austenitic stainless steel have similar strength properties, but the ductility of the latter is much higher than that of other types of stainless steel. (For comparison, the curve of pure iron is also included in the figure).
The corrosion resistance of metal is not only determined by its material, but also by the type, concentration, temperature, pressure, and other environmental conditions of the corrosive medium.
In practical applications, the oxidizing capacity of the corrosive medium has the greatest impact on metal corrosion. Therefore, when selecting stainless steel grades for specific working environments, it is important to consider the characteristics of the corrosive medium.
In weakly corrosive media such as atmosphere, water, and steam, the corrosion resistance of stainless steel can be ensured as long as the Cr content of the solid solution in the stainless steel matrix is greater than 13%. This makes it suitable for use in components like water compressor valves, steam generator turbine blades, and steam pipes.
However, in oxidizing media like nitric acid, the NO3- ions have a strong oxidizing capacity. This results in the formation of an oxide film on the surface of stainless steel with short passivation time, thereby compromising its corrosion resistance.
H+ in acid acts as a cathodic depolarizer. As the concentration of H+ increases, the depolarization of cathode strengthens, and the chromium content required for passivation also increases. Therefore, only the oxide film that contains high chromium exhibits good stability in nitric acid.
In boiling nitric acid, 12Cr13 stainless steel is not corrosion-resistant. However, Cr17 and Cr30 steel with chromium content of 17% – 30% are corrosion-resistant in nitric acid with a concentration of 0% – 65%.
In non-oxidizing media such as dilute sulfuric acid, hydrochloric acid, and organic acid, the oxygen content of such corrosive media is low, and the passivation time needs to be extended. When the oxygen content in the medium is low to a certain extent, stainless steel cannot be passivated. For example, in dilute sulfuric acid, SO42- in the medium is not an oxidant, and the oxygen content dissolved in the medium is relatively low, making it incapable of passivating steel. Consequently, the corrosion rate of chromium stainless steel is even faster than that of carbon steel.
Thus, general Cr stainless steel or Cr Ni stainless steel is difficult to achieve the passivation state, and it is not corrosion-resistant when working in this kind of medium. To improve the passivation ability of steel, elements like molybdenum, copper, and others need to be added.
Hydrochloric acid is a non-oxidizing acid that is known to cause corrosion in stainless steel. To prevent corrosion, a Ni-Mo alloy is required to form a stable protective film on the surface of the alloy.
In strong organic acids, passivation of chromium and chromium-nickel stainless steel is difficult due to low oxygen content in the medium and the presence of H+. The addition of Mo, Cu, Mn, and other elements to the steel can improve its passivation ability. Therefore, Cr-Mn stainless steel is considered a better option.
To make the steel corrosion-resistant and easy to passivate, a certain amount of Mo and Cu are added to the steel.
In mediums containing Cl-, the oxide film on the surface of stainless steel is easily destroyed, leading to pitting corrosion of steel. As a result, seawater is highly corrosive to stainless steel.
It is important to note that no stainless steel can resist corrosion from all types of media. Therefore, the selection of stainless steel must be based on the specific corrosion environment and the characteristics of various types of stainless steel.








