Why does aluminum alloy get anodized, and what happens during the process? Anodizing not only enhances aluminum’s corrosion resistance and aesthetic appeal but also increases its surface hardness. This article will guide you through the science behind anodizing aluminum alloys, the various surface treatment methods involved, and how they impact the final product’s durability and appearance. Dive in to discover the precise techniques and benefits of this essential industrial process.

Low density; good plasticity; easy to strengthen; good conductivity; corrosion-resistant; recyclable; weldable; easy surface treatment.

1) Corrosion properties:
(1) Acidic Corrosion: Aluminum exhibits different corrosion behaviors in various acids. In general, a passivation film is formed in oxidizing concentrated acids, which has excellent corrosion resistance, while in dilute acids, there are “pitting” corrosion phenomena. Localized corrosion;
(2) Alkaline Corrosion: In alkaline solutions, alkali reacts with aluminum oxide to form sodium aluminate and water, which further reacts with aluminum to form sodium aluminate and hydrogen. General corrosion;
(3) Neutral Corrosion: In neutral salt solutions, aluminum can either be passive or corrode due to the effect of certain cations or anions. Pitting corrosion.
2) Forms of corrosion:
Pitting corrosion, galvanic corrosion, crevice corrosion, intergranular corrosion, filiform corrosion, and exfoliation corrosion, etc.
Pitting Corrosion: The most common form of corrosion, the degree of which is related to the medium and the alloy.
Galvanic Corrosion: Contact corrosion, dissimilar (bimetallic) metal corrosion. In an electrolyte solution, when two metals or alloys are in contact (conducting), the corrosion of the more negative metal is accelerated, while the more positive metal is protected from corrosion.
Crevice Corrosion: Occurs when two surfaces are in contact with each other, forming a crevice. The oxygen concentration cell is formed due to the dissolution of oxygen in this area, leading to corrosion within the crevice.
Intergranular Corrosion: Related to improper heat treatment, alloy elements or intermetallic compounds precipitate along the grain boundaries, which act as anodes relative to the grains, forming a corrosion cell.
Filiform Corrosion: A type of underfilm corrosion that develops worm-like under the film. This film can be a paint film, or other coatings, and generally does not occur under the anodic oxide film. Filiform corrosion is related to alloy composition, pretreatment before coating, and environmental factors, including humidity, temperature, and chlorides.
Exfoliation Corrosion: Also known as peeling corrosion.
Surface mechanical pretreatment (mechanical polishing or sanding, etc.), chemical pretreatment or chemical treatment (chemical conversion or chemical plating, etc.), electrochemical treatment (anodization or electroplating, etc.), and physical treatment (spraying, enamel vitrification, and other physical surface modification techniques), etc.
Enamel Vitrification: Melting a mixture of inorganic substances into glass-like materials of different melting points.
Corrosion resistance; hardness and wear resistance; decorative; adhesion of organic coating and electroplated layer; electrical insulation; transparency; functionality.
(1) To enhance good appearance conditions and surface finishing quality.
(2) To improve product grade.
(3) To reduce the impact of welding.
(4) To create decorative effects.
(5) To obtain a clean surface.
(1) Selection of abrasive type and granularity:
This is based on the hardness of the workpiece material, surface condition, and quality requirements; the harder or rougher the surface, the harder and coarser the abrasive used.
(2) Polishing should be carried out in multiple steps, and the pressure of the workpiece towards the grinding wheel should be moderate.
(3) A new grinding wheel should be preliminarily scraped to achieve balance before the abrasive is adhered.
(4) The abrasive should be replaced regularly.
(5) Alloy materials should be selected according to different needs.
(6) The appropriate grinding wheel speed should be selected, generally controlled at 10~14m/s.
(7) The polishing effect depends on factors such as the abrasive, the rigidity of the grinding wheel, the rotation speed of the wheel, the contact pressure between the workpiece and the grinding wheel, practical experience, and skilled techniques.
Grinding: The operation after the cloth wheel is bonded with abrasive. Purpose: To remove burrs, scratches, corrosion spots, sand eyes, pores, and other apparent defects on the workpiece surface.
Polishing: The operation after applying polishing paste on a soft cloth wheel or felt wheel.
Common problem: “Scorch” mark.
Cause:
(1) Improper selection of grinding wheel, abrasive, and polishing agent;
(2) Improper force used in polishing;
(3) Prolonged grinding time;
(4) Overheating during grinding.
Measures:
(1) Slight alkali etching in a dilute alkali solution;
(2) Mild acid etching: such as chromic acid-sulfuric acid solution, or 10% sulfuric acid solution used after heating;
(3) 3wt% Na2CO3 and 2wt% Na3PO4, the solution is treated at a temperature of 40~50℃ for 5min, severe cases can be extended to 10~15min.
After the above cleaning and drying treatment, immediate repolishing should be done using a precision polishing wheel or mirror polishing wheel.
Prevention:
Use appropriate grinding wheels and polishing wheels; use the appropriate polishing agent; the grinding time between the workpiece and the polishing wheel should be properly controlled.
1) Degreasing methods:
Acidic degreasing, alkaline degreasing, and organic solvent degreasing. Purpose: To remove oil, grease, dust, and other contaminants from the surface of the aluminum to allow for a more uniform alkali wash, thereby improving the quality of the anodic oxidation film.
2) Principle
(1) Principle of acidic degreasing: In an acidic degreasing solution based on H2SO4, H3PO4, and HNO3, oils and fats undergo hydrolysis to produce glycerin and corresponding higher fatty acids, achieving the goal of degreasing.
(2) Principle of alkaline degreasing: The alkali reacts with the oil to form soluble soap. This saponification reaction removes the bond between the oil and the surface of the aluminum material, achieving the goal of degreasing.
(3) Principle of organic solvent degreasing: Taking advantage of the fact that oils are readily soluble in organic solvents, both saponified and non-saponified oils can be dissolved. This method has strong degreasing capability, is fast, and non-corrosive to aluminum, thereby achieving the goal of degreasing.

1) Purpose: to remove surface contaminants, completely eliminate the natural oxide film on the aluminum surface, reveal the pure metal base, and prepare for the subsequent main surface treatment process.
2) The three major defects of alkali washing: rough appearance, spots, and streaks.
3) Appearance
(1) Rough appearance: a common problem when producing sandblasted aluminum materials with alkali washing, often caused by structural defects in the original aluminum material (large grains or large intermetallic compound precipitates); improving the internal structure quality of the original aluminum material can solve the problem at the source.
Causes: A: The original grain size of the aluminum rod for extrusion is large. B: The heating temperature of the aluminum rod is too high or the extrusion speed is too fast. C: The tonnage of the extruder used is too small. D: Insufficient quenching after extrusion. E: The alkali washing speed is too fast.
Countermeasures: Use extruded aluminum rods with a grain size that meets national standards; control the exit temperature of extruded products; strengthen quenching after extrusion; reasonably control the alkali washing speed, etc.
(2) Spots: a fatal defect in aluminum surface treatment: interrupt subsequent processes or dispose of as scrap.
Causes:
A: The proportion of recycled aluminum added when melting casting rods is too high. Al2O3 has a melting point as high as 2050°C, it does not melt during smelting, only shatters; the erosion during the alkali washing process leads to snowflake-like corrosive spots. Countermeasures: Control the proportion of recycled aluminum in the anodic oxidation film, it should be less than 10%; refining and slag removal of the melt, the melt should rest for about 25min before casting and the melt should be filtered, etc.
B: The chlorine ion content in the water is high. When the material quality of the aluminum material is poor and the chlorine ion content of the used water is also high, corrosive spots will be revealed during alkali washing or water washing before and after alkali washing. Countermeasures: Improve the quality of the original aluminum material; use tap water that meets national standards; use nitric acid or nitric acid plus sulfuric acid for descaling; adding 1~5g/L HNO3 to the water tank nickel can also effectively suppress the corrosive effect of chloride ions.
C: Atmospheric corrosion. Aluminum materials placed in coastal atmospheric environments for about 3 days, next to corrosive atmosphere smelting furnaces, rainy weather, etc., often have corrosive marks or spots on the surface. Countermeasures: Shorten the cycle time of the original aluminum material into anodic oxidation; place the original aluminum material with anodic oxidation in a dry and well-ventilated environment; for long-term placement or rainy days, appropriate cover treatment can be performed on the original aluminum material, etc.
D: Extrusion “hot spot”. The aluminum material comes into contact with the thermally conductive graphite roller on the discharge table, due to different local cooling speeds, the precipitation phase (Mg2Si phase, temperature range 400~250℃) forms in the aluminum material, presenting interval spots. Countermeasures: Control the operation speed of the extrusion discharge table (should be greater than the extrusion speed of aluminum); use other heat-resistant materials with poor thermal conductivity to replace graphite rollers; borrow the quenching strength of gun wind; quickly reduce the extrusion outlet aluminum material to below 250℃.
(3) Streaks: Defects of alkali washing streaks caused by improper alkali washing process conditions and operations (alkali washing speed is too fast and transfer speed is too slow). Countermeasures: A: Speed up the transfer. B: Lower the temperature of the alkali washing bath. C: Reduce the NaOH concentration in the bath. D: The aluminum material is too densely packed, it should be reduced appropriately.
Purpose: To remove surface dust, prevent contamination of the subsequent anodizing bath, and improve the quality of the oxide film.
Methods: Nitric acid dust removal, sulfuric acid dust removal,
Fluoride sand surface treatment is an acid corrosion process that uses fluoride ions to produce highly uniform and high-density point corrosion on the surface of aluminum.
Defects and countermeasures:
(1) Surface has blemishes: When there are too many precipitates in the tank and the concentration of fluoride ions is low, the reaction strength is weak. Precipitates deposit or stay too long on the surface, hindering the normal corrosion of fluoride ions.
Countermeasure: Remove excessive precipitates in the tank, reduce the density of aluminum, add a suitable amount of ammonium bifluoride and additives, increase the concentration of fluoride ions, and increase the reaction strength.
(2) The surface is not easy to sand: The tank liquid is contaminated by the preceding acid degreasing, causing the PH to decrease, and the concentration of fluoride ions and additives is insufficient.
Countermeasure: Adjust the PH value with ammonia or ammonium fluoride, and add ammonium bifluoride and additives, etc.
(3) The sand grains on the surface are too coarse: The concentration of fluoride ions in the tank is too high or the additives are insufficient, or the treatment time is too long.
Countermeasure: Take corresponding measures to control.
(4) Surface gloss varies: The tank process conditions are not properly controlled, or the choice of additives is inappropriate, or there is a problem with the aluminum material.
Countermeasure: Take corresponding measures to control.
(5) Partial areas do not sand: There is a composite oxide film in the local area.
Countermeasure: Adjust the process flow, such as polishing, buffing, re-acid washing or alkali washing, etc.
1) Chemical Polishing: By controlling the selective dissolution of the aluminum surface, the microscopic protrusions dissolve faster than the recesses, achieving a smooth and bright surface.
2) Electrochemical Polishing, also known as electropolishing. The principle is similar to chemical polishing, relying on the selective dissolution of the protruding parts of the surface to achieve smoothness. The difference is the application of an external current, which shortens the processing time.
3) Common point: Both use the same polishing mechanism; Difference: Electrochemical polishing applies a current during the process, while chemical polishing uses chemical oxidants.
Compared with mechanical polishing, chemical and electrochemical polishing have the following advantages:
(1) Simple equipment, easy to control process parameters, cost-saving, and a brighter surface;
(2) Capable of processing large components or large quantities of small components, as well as complex-shaped workpieces;
(3) Cleaner surface, no residual mechanical polishing dust, with good corrosion resistance;
(4) The mirror reflectivity of the chemically polished surface is higher, the metal texture is better, and no powdery “frost” forms on the surface.
1) Defects and Countermeasures of Chemical Polishing (Taking Phosphoric Acid-Sulfuric Acid-Nitric Acid Process as an Example)
(1) Insufficient brightness: Influenced by the composition of aluminum, the content of nitric acid, etc.
Countermeasure: Use high-purity aluminum, control the concentration of nitric acid, and ensure the aluminum is dry before polishing.
(2) White deposits: Excessive dissolution of aluminum, which requires control of its content in the bath.
Countermeasure: Adjust the amount of dissolved aluminum in the bath to within the normal range.
(3) Rough surface: Too high nitric acid content, overly intense reaction; or too high Cu content.
Countermeasure: Strict control of nitric acid content; improve the internal quality of the material, reduce the amount of additives, etc.
(4) Transfer corrosion: Occurs when the transition to the rinsing process after chemical polishing is slow.
Countermeasure: Transfer to water for rinsing promptly.
(5) Pitting corrosion: Occurs due to the accumulation of gas on the surface forming gas pockets; or due to low nitric acid or Cu content.
Countermeasure: Load the parts properly, increase the tilt of the workpiece, enhance stirring to allow gas escape. Clean the surface thoroughly; control the nitric acid content, etc.
2) Defects and Countermeasures of Electrochemical Polishing (taking phosphoric-sulfuric-chromic acid process as an example)
(1) Electrical Burns: caused by insufficient conductive surface area, poor contact, overly rapid increase in voltage, or excessive current density. Countermeasure: Ensure good contact between the workpiece and the electrical fixture, sufficient contact area to accommodate high current, and avoid overly rapid voltage increase.
(2) Dark Spots: caused by low current density or uneven local distribution of power lines. Countermeasure: Avoid overloading, and aim to prevent dead zones where power lines can’t reach.
(3) Gas Stripes: caused by gas escaping. Countermeasure: Position every surface of the workpiece at an angle during loading, place decorative surfaces vertically towards the cathode, and avoid gas accumulation.
(4) Ice Crystal-like Adherences: formed by high aluminum content in the bath or high phosphoric acid content creating aluminum phosphate precipitate. Countermeasure: Reduce the amount of dissolved aluminum in the bath, or lower the phosphoric acid content.
(1) Barrier type: Also known as shield type or blocking layer oxide film, it is closely adjacent to the metal surface, dense, poreless, thin, with a thickness determined by the oxidation voltage, not exceeding 0.1μm, mainly used for electrolytic capacitors.
(2) Porous type: Composed of two layers of oxide film, the bottom layer is a blocking layer, with a dense, poreless thin oxide layer structure identical to the barrier film, the thickness is voltage-dependent; the main part is a porous layer structure, the thickness of which depends on the amount of electricity passed through.
(Blocking layer: Refers to the oxide layer with barrier film properties and formation rules that separate the porous layer of the porous oxide film from the aluminum metal.)
Porous anodic oxide film composition: blocking layer and porous layer; the structure and formation rules of the blocking layer are equivalent to the barrier type oxide film; the generation rules, structure and composition of the porous layer are completely different from the blocking layer.
1) Thickness of the blocking layer: It depends on the externally applied oxidation voltage and is not related to the oxidation time. The film formation rate or film ratio δb/Va; the film formation rate of the barrier oxide film is greater than the film formation rate of the blocking layer of the porous oxide film.
Thickness of the porous layer: total thickness = porous layer + blocking layer; total thickness is directly proportional to the product of current density and oxidation time (i.e., the amount of electricity passed through).

2) Composition of the blocking layer: dense, poreless amorphous oxide.
Composition of the porous layer: amorphous Al2O3, but not pure.
3) Structure of the blocking layer: double layer structure. Outer layer: contains solution anions; inner layer: composed mainly of pure aluminum oxide.
Structure of the porous layer: outer layer: contains γ-Al2O3 and α-AlOOH; inner layer: amorphous Al2O3, water infiltration into the oxide film gradually transforms into boehmite α-AlOOH.
Impacts of the parameters in the aluminum sulfuric acid anodizing process
(1) The influence of sulfuric acid concentration:
It affects the thickness of the oxidation film barrier layer, the conductivity of the electrolyte, the dissolving effect on the oxidation film, the corrosion resistance of the oxidation film, and the quality of subsequent pore sealing.
A high concentration has a significant dissolving effect on the oxidation film, resulting in a thin barrier layer and a decrease in the voltage required to maintain a certain current density; the reverse results in a thick film and high voltage.
A high concentration of sulfuric acid requires low voltage to maintain a certain current, but it has a significant impact on the oxidation film. As the concentration and temperature of sulfuric acid increase, the required voltage decreases.
However, a higher sulfuric acid concentration increases the acid’s erosion of the oxidation film. As the concentration of sulfuric acid increases, efficiency decreases: i.e., more electricity is consumed to obtain an oxidation film of a certain thickness. As the concentration of sulfuric acid increases, the corrosion resistance and wear resistance of the film decrease.

(2) The influence of bath temperature:
1) When the bath temperature increases within a certain range, the type of oxidation film obtained decreases, the film becomes softer but brighter;
2) When the bath temperature is high, the pore diameter and taper of the outer layer of the oxidation film tend to increase, making sealing more difficult, and it is also prone to sealing “frosting”.
3) The oxidation film obtained at higher bath temperatures is easy to dye, but it is difficult to maintain the consistency of color depth, and the oxidation temperature of the general dyed film is 20~25℃;
4) The oxidation film obtained by lowering the bath temperature has high hardness and good wear resistance, but maintaining the same current density during maintenance requires a higher voltage, and the common film uses 18~22℃.
For films thicker than 15μm, when the bath temperature rises, the film quality and metal loss ratio significantly decrease, and the hardness of the outer layer of the film is lower.
Temperature significantly affects the quality of the oxidation film: temperatures above 15℃ all produce non-crystalline soft films. Lower temperatures help produce dense oxidation films. As the temperature rises, the hardness of the film decreases.
To obtain a film with high hardness and good wear resistance, low-temperature anodizing must be used. Except for 3004 alloy, generally, alloys have the best corrosion resistance at 20℃. Corrosion resistance decreases as the temperature rises and drops to its lowest at 40℃.
(3) Influence of oxidation voltage:
The voltage determines the size of the pores in the oxidation film: low voltage – small pore size, more pores – large pore size, fewer pores.
(Within a certain range, high voltage is conducive to the formation of dense, uniform oxidation films. Under constant voltage, the current density decreases as the oxidation time increases.
The higher the voltage required to maintain a certain current, the more heat is released during the oxidation process, which is not conducive to the stability of the oxide film performance. When the current is constant, the lower the temperature, the higher the voltage.)
(4) Influence of oxidation current:
The oxidation current directly affects production efficiency: high current production efficiency is high.
(High current requires a large capacitance capacitor, resulting in significant fluctuations in film thickness and easily causing “burns” to the workpiece. Under low current, the oxidation time is long, which reduces the corrosion resistance and wear resistance of the film. The optimal current is 1.2~1.8A/dm2.
The higher the concentration of sulfuric acid, the better the conductivity of the bath solution, and the greater the current density under the same voltage. As the aluminum content increases, the resistance of the bath solution increases, and its conductivity decreases.)
(5) The influence of bath solution stirring:
In order to make the anodic oxidation bath solution temperature and concentration uniform, especially when using a larger current, a large amount of heat is generated at the film-bath solution interface, and stirring reduces the interface temperature.
(6) The influence of oxidation time:
Under constant current oxidation, the increase in oxidation film thickness is directly proportional to time within a certain period. (Based on electrolyte concentration, bath solution temperature, current density, oxidation film thickness, and performance requirements, etc.)
1) Sulfuric acid process: Low production cost; high film transparency; good corrosion and wear resistance; easy electrolytic and chemical coloring.
2) Chromic acid process: The oxide film thickness is average, with a rough surface; the film is soft; it has less wear resistance than the sulfate film, but it has good elasticity.
3) Oxalic acid process: The oxide film has low porosity, better corrosion resistance, wear resistance, and electrical insulation than the sulfuric acid film, but it has a higher cost.
4) Phosphoric acid process: The oxide film is thinner, with larger pores.
1) AC: Low current efficiency; poor corrosion resistance of the oxide film, low hardness.
2) DC: High production cost; high film transparency; good corrosion and wear resistance; easy electrolytic and chemical coloring.
Mainly affects the wear resistance, corrosion resistance, brightness, and electrolyte conductivity of the oxide film
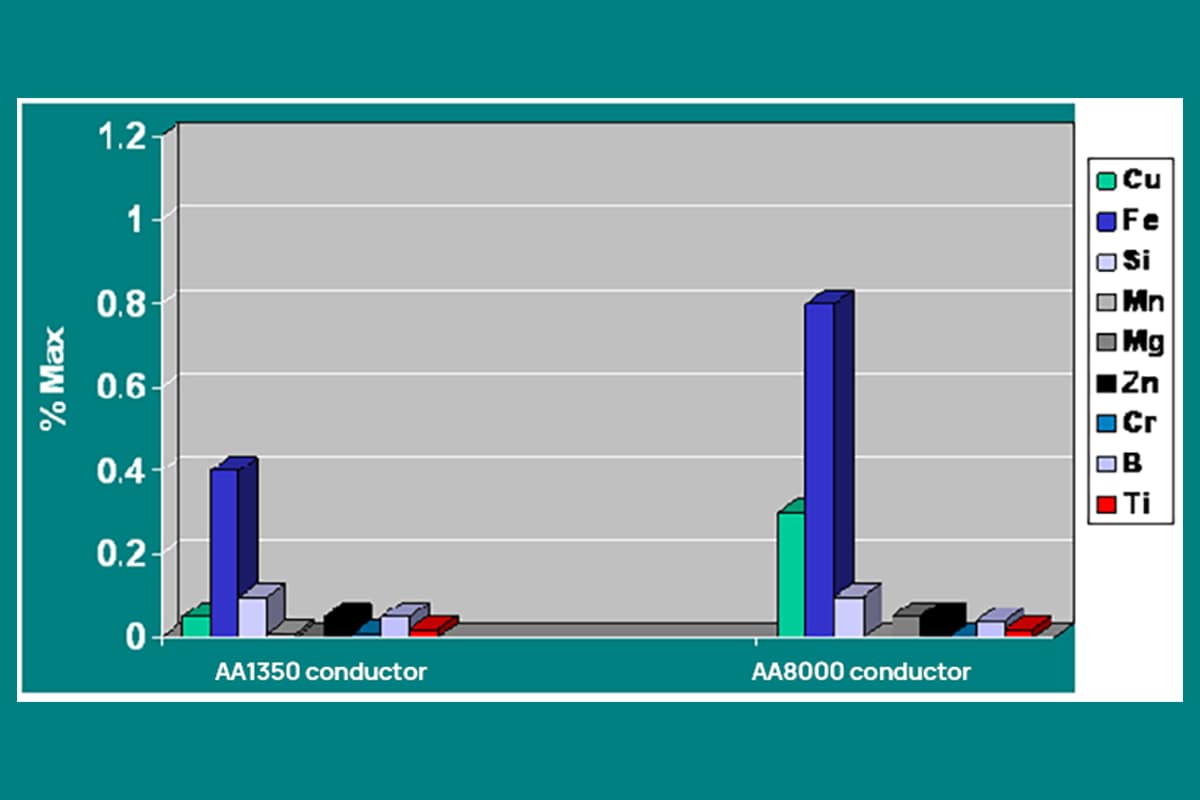
(1) Aluminum ions:
A concentration of 1~10g/L is beneficial, but more than 10g/L will cause an impact. The current decreases as the concentration of aluminum ions increases; coloring becomes more difficult; when the aluminum content is high, insoluble aluminum salts are deposited on the surface of the aluminum workpiece, tank wall, and heat exchanger, affecting the appearance of the product and the efficiency of heat exchange.
(2) Cations Fe, MN, Cu, and Ni, etc.:
Fe: Harmful impurity, mainly comes from sulfuric acid and aluminum. When the Fe content exceeds 25~50μg/g, the oxide film encounters many problems, such as decreased brightness, and soft film.
Mn: The effect is similar to Fe, but not as significant.
Cu and Ni: Mainly come from alloyed aluminum, their effects are similar, when the content exceeds 100μg/g, the corrosion resistance of the oxide film decreases.
(3) Anions such as phosphate, nitrate, chloride, etc.:
Phosphate: Caused by insufficient washing after chemical polishing; the effect is not significant when the content is low (ppm level). The main hazard when the content is high is that the phosphate is adsorbed by the oxide film and released during water sealing, which will harm the sealing quality when it exceeds 5μg/g.
Nitrate: Mainly comes from insufficient washing after the previous process and commercial sulfuric acid in the bath. When the content exceeds 30μg/g, it is detrimental to brightness, and too high will increase the bath’s dissolving ability, which is not conducive to film formation.
Chloride: Mainly comes from the water used, the chloride content in tap water is high. When Cl- and F- exceed 50μg/g, the oxide film produces corrosion spots.
The preparation of a hard anodic oxide film has no fundamental difference from ordinary anodization in terms of principles, equipment, and processes. The specific technical measures are slightly different. The difference lies in reducing the dissolution rate of the oxide film during the oxidation process.
The hard anodic oxide film has a greater thickness, higher hardness, better wear resistance, lower porosity, higher dielectric breakdown voltage, but the surface smoothness is slightly worse.
(When the applied voltage is high, concentration is low, and treatment time is long, the film will be thick, hard, wear-resistant, have high dielectric breakdown voltage, low porosity, large pore size, and poor surface smoothness.)
(1) Low bath temperature: less than 5°C, the lower the temperature, the harder the film. The bath temperature for ordinary sulfuric acid anodization is around 20°C.
(2) Low bath concentration: generally less than 15% for sulfuric acid; the bath concentration for ordinary anodization is around 20%.
(3) Addition of organic acids to the sulfuric acid bath: oxalic acid, tartaric acid, citric acid, etc.
(4) High applied current/voltage: 2~5A/dm2, 25~100V. Ordinary anodization uses 1.0~1.5 A/dm2, below 18V.
(5) Gradual voltage increase operation method: step-by-step pressurization.
(6) Use of pulse power supply or special waveform power supply: for high Cu alloy or high Si cast aluminum alloy.
1) Sn salt electrolytic coloring process:
This mainly involves single Sn salt and Sn-Ni mixed electrolytic coloring, with SnSO4 being the primary coloring salt. The color is achieved through the reduction of Sn2+ ions in the micropores of the anodized film.
Advantages: Sn salt has good impurity resistance, strong electrolytic coloring solution distribution ability, and simple industrial control. There are no inherent difficulties with alternating current Sn salt coloring. Disadvantages: Sn2+ has poor stability, and it is difficult to control color differences and hues.
2) Ni salt electrolytic coloring process:
Similar to the Sn salt electrolytic coloring process, it involves the deposition of Ni for coloring. Advantages: Ni salt coloring is fast, and the bath solution has good stability. Disadvantages: It is sensitive to impurities in the bath solution.
1) AC coloring.
Advantages: It overcomes the risk of oxide film peeling off in DC electrolytic coloring. Disadvantages: In AC coloring, the anode voltage affects the speed of the cathode coloring reaction, causing a decrease in anode current density and cathode current density, thereby slowing down the coloring speed.
2) DC coloring.
Advantages: Fast coloring speed, high electrical energy utilization rate. Disadvantages: There is a risk of oxide film peeling off in DC electrolytic coloring.

(1) The anodized film of aluminum obtained in sulfuric acid solution is colorless and porous;
(2) The oxide film should have a certain thickness, which should be more than 7um;
(3) The oxide film should have certain porosity and adsorption;
(4) The oxide layer should be complete and uniform, without defects such as scratches, sand eyes, or spot corrosion;
(5) The oxide film itself should have an appropriate color and no metallographic structure differences, such as uneven grain size or severe segregation.
(1) Organic dyeing is based on the theory of material adsorption, including physical adsorption and chemical adsorption.
Physical adsorption: Molecules or ions are adsorbed by electrostatic force. The composition of the oxide film is amorphous aluminum oxide, the dense barrier layer near the aluminum substrate is on the inside, and the porous structure that grows outward in a bell shape is on top, showing excellent physical adsorption performance. When dye molecules enter the film pores, they are adsorbed onto the pore walls.
Chemical adsorption: Adsorption by chemical force. At this time, the organic dye molecules react chemically with the aluminum oxide and exist within the film pores due to chemical bonding.
This type of adsorption includes the following: the oxide film forms a covalent bond with the sulfonic group on the dye molecule; the oxide film forms a hydrogen bond with the phenolic group on the dye molecule; the oxide film forms a complex with the dye molecule.
(2) Inorganic dyeing mechanism: During dyeing, the oxidized workpiece is first immersed in an inorganic salt solution in a certain order, and then successively immersed in another inorganic salt solution, causing these inorganics to undergo a chemical reaction in the film pores to form a water-insoluble colored compound. This fills the oxide film pores and seals them, thus giving the film layer color.
Process flow: Pre-treatment – Anodization – Cleaning – Ammonia neutralization or other processing – Cleaning – Dyeing – Cleaning – Sealing treatment – Drying.
Standards:
1) The concentration for easy dyeing: Light colors are generally controlled at 0.1~1g/L, while dark colors require 2~5 g/L, and black requires over 10 g/L;
2) Temperature of the dye solution: Generally controlled at 50~70℃;
3) PH value of the dye solution: PH range is 5~6;
4) Dyeing time: Usually between 5~15min.
(1) Effect of sodium sulfate: Sodium sulfate slows down the dyeing rate, this effect increases with the increase of sulfur groups in the dye ions, especially in metal complex dyes.
(2) Effect of sodium chloride: The main cause of pitting (white spots). Pitting is suppressed by cathodic current.
(3) Effect of surfactants: Non-ionic surfactants have no effect on dyeing, but cationic surfactants such as in black MLW will slow down the dyeing, therefore ionic surfactants are not suitable to be added to the degreaser as some anions are not conducive to dyeing.
(4) Effect of trivalent aluminum ions: A small amount of Al3+ has no effect on many dye solutions, unless it reaches 500~1000ug/g, which may cause color change, such as blue turning red, etc.
(5) Effect of heavy metal ions.
(6) Effect of anions.
(7) Effect of bacterial action on dyeing: Bacteria proliferate in the dye solution, making the dye solution moldy. Initially, small bubbles appear on the surface of the dye solution. When the dye solution is left standing without working, some insoluble colored particles gather around the bubbles, causing abnormal dyeing.
If visible to the naked eye, the moldy substance suspended on the surface should be removed and an appropriate bactericide, like dichlorophenol G4, should be added at 0.05~0.10g/L, dissolved in an ethanol solution and added to the tank.
Sometimes it is necessary to dump the dye solution. At this time, use a bactericide or hypochlorous acid solution to clean the tank wall, and then reconfigure.
(8) Effect of insoluble impurities on dyeing: The dye solution sometimes inevitably carries oil stains, contaminating the workpiece and causing the dyeing to blossom.
At this time, oil-absorbing paper should be used to absorb and remove it, or a small amount of non-ionic surfactant should be added to disperse the oil droplets so that they do not gather on the surface of the dye solution.
Generally operated at room temperature, usually in two steps: first immerse in the first solution for 5~10 minutes, then rinse and immerse in the second solution for another 5~10 minutes to achieve the desired color.
Common inorganic dyeing process standards.
| Colors | Solution Components: | Concentration/(g/L) | Production of Colored Salts |
| Blue | ① [K4Fe(CN)6.3H2O] ② [FeCl3] or [Fe2(SO4)2] | 30~50 40~50 | Ferrous Ferricyanide (Prussian Blue) |
| Black | ① [CoAc2] ② [KMnO4] | 50~100 15~25 | Cobalt Oxide |
| Yellow | ① [PbAc2.3H2O] ② [K2Cr2O7] | 100~200 50~100 | Lead Chromate |
| White | ① [PbAc2.3H2O] ② [Na2SO4] | 10~50 10~50 | Lead Sulfate |
| Brown | ① [K3Fe(CN)6] ② [CuSO4.5H2O] | 10~50 10~100 | Copper Ferrocyanide |
| Gold | [NH4Fe(C2O4)2](Ph=4.8~5.3, 35~50oC, 2min) | 10 (Shallow) 25 (Deep) |
1) Color does not apply.
Solution:
a) Change the pigment
b) Adjust the PH
c) Increase the film thickness
d) Dye in time
e) Choose the right pigment.
2) Some areas do not take color or the color is light.
Solution:
a) Strengthen protective measures
b) Increase pigment concentration
c) Increase film thickness
d) Clamp workpiece, adjust position
e) Change dye solution
f) Improve pigment dissolution.
3) The surface appears white and foggy after dyeing.
Solution:
a) Remove water vapor
b) Adjust the fading solution concentration
c) Shorten the fading time.
4) The color blooms after dyeing.
Solution:
a) Adjust the PH and enhance cleaning
b) Improve pigment dissolution
c) Lower dye solution temperature.
5) There are spots after dyeing.
Solution:
a) Rinse the surface of the sample with water
b) Filter the dye solution
c) Place the workpiece in a water tank after oxidation
d) Enhance protection.
6) The color fades easily after dyeing.
Solution:
a) Increase PH
b) Raise the dye bath temperature, extend dyeing time, adjust the sealing bath PH, extend sealing time.
7) The dyed surface can be easily rubbed off.
Solution:
a) Re-oxidize
b) Increase dye solution temperature
c) Increase oxidation temperature.
8) The color is too dark after dyeing.
Solution:
a) Dilute the dye solution
b) Lower the temperature
c) Shorten the time.
1. Sealing
A chemical or physical process performed on the oxidized film after aluminum anodization to reduce its porosity and adsorption capacity.
The main principles of sealing include:
(1) hydration reaction; (2) inorganic filling; (3) organic filling.
2. Technique of thermal sealing
The technique of thermal sealing is accomplished through the hydration reaction of aluminum oxide, transforming amorphous aluminum oxide into a hydrated aluminum oxide known as boehmite, or Al2O3•H2O(AlOOH).
The essence of the thermal sealing mechanism is the hydration reaction, often referred to as “hydration-thermal sealing”.
3. The role of the hydration reaction
It causes a volume expansion of 30%, the increased volume fills and seals the micropores of the oxidized film, thereby enhancing its anti-pollution and corrosion resistance while decreasing conductivity (increasing impedance), and enlarging the dielectric constant.
4. The influence of impurities in water
1) The efficiency of sealing significantly depends on the quality of water and PH control;
2) Common impurities include SiO2 and H2SiO3; 3) Countermeasures: ion exchange.
5. Comparison of boiling water sealing parameters and cold sealing parameters
1) The temperature of boiling water sealing: generally above 95 degrees. Cold sealing is at room temperature.
2) The PH value of boiling water sealing: the optimal range is 5.5~6.5. Cold sealing range is also 5.5~6.5, with an industrial control best at 6.
3) The time of boiling water sealing: depends on the film thickness, pore size, and sealing quality test requirements. Cold sealing generally stipulated as 10~15 minutes.
1) Appearance and color difference:
Inspection methods: Visual and instrument detection.
Pros and cons: Visual inspection is simple, but it is easily affected by the shape and size of the sample and the intensity of the light. Instrument detection solves the shortcomings of visual inspection and is suitable for measuring the color of reflected light.
2) Oxide film thickness:
Measurement methods:
a) Cross-sectional thickness microscopic measurement method: Film thickness greater than 5um, vertical.
b) Spectral beam microscope measurement method: Film thickness greater than 5um, oxide film refractive index 1.59~1.62.
c) Mass loss method: Film thickness less than 5um, dissolution method, surface density, oxide film density (sulfuric acid liquid oxidation) before and after sealing are 2.6 and 2.4g/cm3.
d) Eddy current method: Not suitable for thin films.
3) Sealing quality:
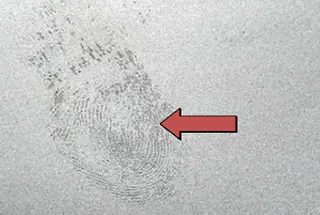
a) Fingerprint test.
b) Quality of dyed spots after acid treatment, not suitable for content with Cu higher than 2% and Si higher than 4%.
c) Phosphochromic acid experiment.
4) Corrosion resistance:
a) Salt spray corrosion test.
b) SO2 humid atmosphere corrosion test.
c) Machu corrosion test.
d) Humid heat corrosion test.
e) Dropping alkali corrosion test.
5) Chemical stability:
a) Acid resistance test.
b) Alkali resistance test.
c) Mortar resistance test.
6) Weather resistance:
a) Natural exposure test.
b) Artificial accelerated weathering test.
7) Hardness:
a) Indentation hardness.
b) Pencil hardness.
c) Microhardness.
8) Abrasion resistance:
a) Abrasion resistance detected by a sandblasting tester.
b) Abrasion resistance detected by a wheel wear tester.
c) Abrasion resistance detected by a falling sand tester.
9) Adhesion:
a) Grid cut experiment.
b) Instrument experiment: Scratch method.
10) Mechanical properties:
a) Impact resistance.
b) Bending resistance.
c) Fatigue performance.
d) Bonding strength.
e) Resistance to deformation fracture.
f) Heat crack resistance.
11) Electrical insulation: Breakdown voltage method.
12) Reflective performance.
13) Others:
a) Coating polymerization performance.
b) Boiling water resistance.
c) Machinability.
Alloy composition, film thickness, curing conditions of high polymer coatings, anodizing conditions, and sealing conditions, etc.