Imagine a metal so versatile that its structure can transform the properties of steel. Austenite, with its unique face-centered cubic structure, plays a crucial role in metallurgy. This article delves into the formation, properties, and applications of austenite, explaining how its behavior impacts everything from toughness to corrosion resistance. Discover how heating temperatures, alloying elements, and original tissue affect austenite’s formation and growth, providing insights into optimizing steel for various industrial applications. Join us to unlock the secrets behind this fascinating metallurgical phase.
English Name: austinite; the name comes from: William Chandler Roberts-Austen, a British metallurgist
Letter code: A, γ.
Definition: solid solution formed by carbon and various chemical elements in γ-Fe.
Features:
Austenite (γ-Fe) has a face centered cubic structure with a maximum void of 0.51 × 10-8cm, slightly smaller than the carbon atom radius, so its carbon dissolving capacity is greater than that of α-Fe.
At 1148 ℃, the maximum dissolved carbon content of γ-Fe is 2.11%.
With the decrease of temperature, the dissolved carbon capacity gradually decreases.
At 727 ℃, the dissolved carbon content is 0.77%.
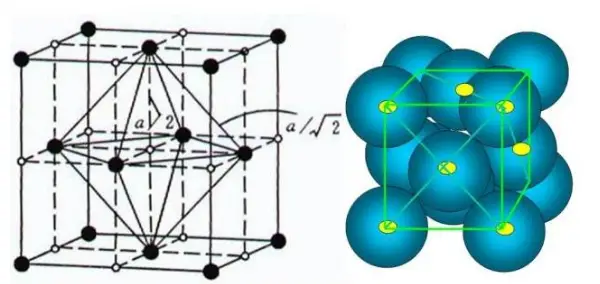
Face centered cubic structure
(1) Low yield strength and hardness
(2) High plasticity and toughness
(3) High thermal strength
(1) Small specific volume, physical performance
(2) Poor thermal conductivity
(3) Large linear expansion coefficient
(4) Paramagnetism
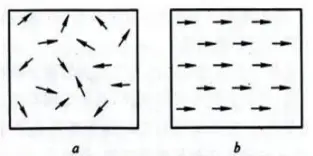
(a) Paramagnetism; (b) Ferromagnetism
Spontaneous arrangement of atomic magnetic moments in a small region.
(1) Application performance of deformation forming
(2) Corrosion resistance of austenitic stainless steel
(3) Sensitive element of expansion instrument

Thermodynamic conditions for Austenite Formation: there is undercooling or superheating T.
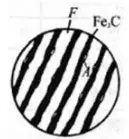
The nucleation of austenite is diffusion type phase transformation.
Nucleation can be formed on the interface between ferrite and cementite, pearlite and austenite.
These interfaces are easy to satisfy the three fluctuation conditions of nucleation energy, structure and concentration.
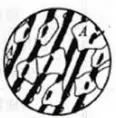
When heated to the austenite phase region, at high temperature, carbon atoms diffuse rapidly, iron atoms and replacement atoms can fully diffuse, both interface diffusion and body protection can be carried out.
Therefore, the formation of austenite is a diffusion type phase transformation.
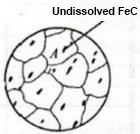
After the ferrite disappears, when the ferrite is kept or heated at t1 temperature, the residual cementite continuously dissolves into the austenite as the carbon continues to diffuse in the austenite.
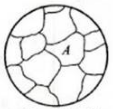
When the cementite has just been completely separated into austenite, the carbon concentration in austenite is still uneven.
Only after a long time of heat preservation or continuous heating, and the carbon atoms continue to fully diffuse, can the austenite with uniform composition be obtained.
Note: there are some differences in the austenite nucleation process of various steels.
In addition to the basic process of Austenite Formation, there are also the dissolution of pre eutectoid phase and the dissolution of alloy carbide in the austenitizing process of hypoeutectoid steel, hypereutectoid steel and alloy steel.
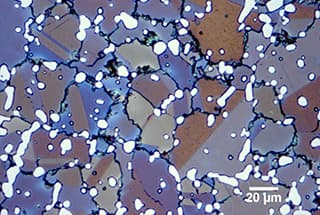
The size of original austenite grain has a great influence on the mechanical properties and technological properties of metal materials.
50 ml of distilled water, 2-3 g of picric acid and 1-2 drops of detergent.
Heat the prepared reagent to about 60 ° C, and then put the sample into erosion for 10-15 minutes.
At this time, the surface of the sample has become black.
Take out and wipe the black film on the surface of the sample with degreasing cotton until it is gray, and dry it for observation.
If the corrosion is too shallow, the corrosion can be continued; If the corrosion is too deep, gently polish it.
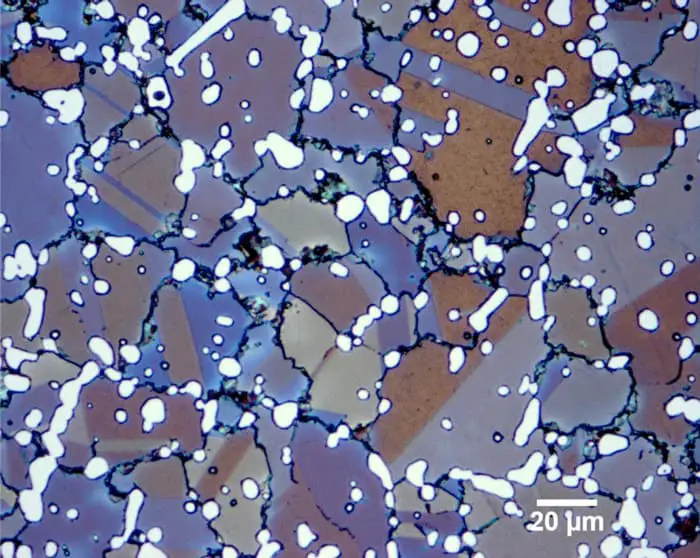
Note: for some samples whose original austenite grain boundaries are difficult to be displayed, erosion polishing, re erosion, re polishing and repeated several times are required.
The time of erosion and polishing is shorter than that of each time until satisfactory.
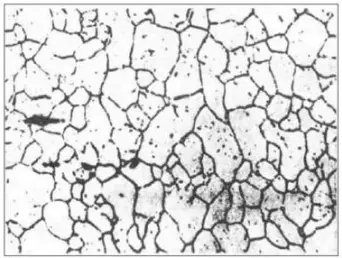
Grain boundary of original austenite in 40Cr quenched state
With an increase in the heating temperature, the diffusion rate of atoms rapidly accelerates, leading to an increase in the austenitizing speed and shortening of the forming time.
The faster the heating speed, the shorter the incubation period becomes. This also results in an increase in the temperature at which austenite starts to transform and the temperature at which the transformation ends. Furthermore, it reduces the amount of time required for the transformation to complete.
Cobalt and nickel have the effect of speeding up the austenitizing process, while chromium, molybdenum, and vanadium have the effect of slowing it down. On the other hand, silicon, aluminum, and manganese do not have any effect on the bainization process of austenite alloy elements.
It is worth noting that the diffusion speed of alloy elements is much slower compared to that of carbon. As a result, the heating temperature for heat treatment of alloy steel is usually higher, and the holding time is longer.
When the cementite in the original structure is in flake form, the speed of austenite formation is faster. Additionally, the smaller the spacing between the cementite particles, the faster the transformation speed.
The original austenite grain also has a larger carbon concentration gradient, which results in a faster growth rate of the grain.
Furthermore, spheroidized annealed granular pearlite has fewer phase interfaces, which makes the austenitization process the fastest among all.
① Within a certain range of carbon content, an increase in carbon content in austenite leads to an increase in grain growth tendency. However, if the carbon content exceeds a certain level, the growth of austenite grains will be hindered.
② The addition of elements such as titanium, vanadium, niobium, zirconium, and aluminum to steel can result in the production of fine-grained steel. This is because carbides, oxides, and nitrides are dispersed along the grain boundaries, which can inhibit grain growth. On the other hand, manganese and phosphorus have the effect of promoting grain growth.
③ Elements that form strong carbides, when dispersed in austenite, can hinder the growth of austenite grains. On the other hand, non-carbide forming elements such as silicon and nitrogen have little effect on the growth of austenite grains.
The growth of austenite grain is closely linked to atomic diffusion in the heating temperature system. As a result, the higher the temperature or the longer the holding time at a specific temperature, the coarser the austenite grain becomes.
The faster the heating speed, the higher the superheat, and the higher the actual formation temperature of austenite. This results in an increase in the nucleation rate, which is greater than the growth rate and makes the austenite grain finer.
In the manufacturing process, rapid heating and short-term heat preservation are often employed to obtain ultra-fine grain structures.
As a general rule, the finer the original structure of steel, the greater the dispersion of carbides, which leads to a finer grain structure of austenite.