Have you ever wondered about the science behind joining metals without melting them? Brazing is a fascinating process that connects metals using a filler material heated to a specific temperature range. In this article, we’ll dive into the world of brazing, exploring its characteristics, advantages, and various types. Join us as we uncover the secrets of this essential metalworking technique and discover how it shapes the products we use every day.

Brazing is a high-temperature joining process where a filler metal is heated to a temperature typically exceeding 450°C (842°F). This temperature is carefully selected to be above the liquidus point of the filler metal but below the solidus temperature of the base materials being joined.
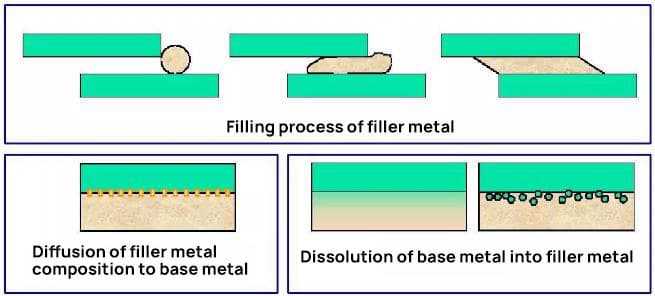
During the brazing process, the molten filler metal wets the surface of the base metals, facilitated by proper flux application or controlled atmosphere. Capillary action then draws the liquid filler into the narrow joint clearance. As the assembly cools, the filler metal solidifies, creating a strong metallurgical bond between the base materials.
This versatile technique allows for joining similar or dissimilar metals, producing high-strength connections with excellent thermal and electrical conductivity. Brazing is widely used in industries such as aerospace, automotive, and HVAC for its ability to create leak-tight joints and join complex assemblies with minimal distortion.

(1) The melting point of the filler metal is substantially lower than that of the base metal, typically by at least 50°C (90°F). This temperature differential ensures that the base metal remains in a solid state during the brazing process, preserving its structural integrity and mechanical properties.
(2) The composition of the filler metal is distinctly different from that of the base metal. This compositional variance is engineered to achieve specific metallurgical properties, such as improved wettability, enhanced flow characteristics, and compatibility with the base metal for optimal joint strength.
(3) The molten filler metal is drawn into and retained in the joint gap between the base metal components through a combination of wetting action and capillary force. This phenomenon, known as capillary action or capillary flow, is governed by factors including surface tension, viscosity, and the gap clearance, typically ranging from 0.025 to 0.125 mm (0.001 to 0.005 inches) for optimal results.
(4) The metallic bond is established through the mutual diffusion of atoms at the interface between the liquid filler metal and the solid base metal. This diffusion process creates an intermetallic layer, which is crucial for the formation of a strong, continuous metallurgical bond. The extent and nature of this diffusion zone significantly influence the joint’s mechanical properties and corrosion resistance.
Decomposition of brazing process
Brazing Advantages:
Brazing Disadvantages:
1) Classification by Solder Melting Point
2) Classification by Brazing Temperature
3) Classification by Heat Source
4) Classification by Atmosphere
5) Classification by Filler Metal Application
6) Classification by Joint Design
Liquidus: The lowest temperature at which the brazing filler metal is completely liquid.
Solidus: The highest temperature at which the brazing filler metal is completely solid.
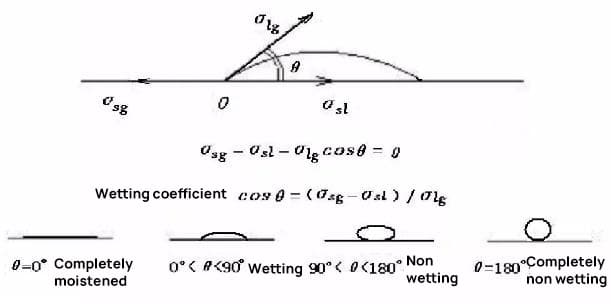
Wetting:
Wetting is the ability of a liquid to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. It is a crucial phenomenon in brazing, encompassing:
In its free state, a liquid tends to form a spherical shape due to surface tension. When a liquid contacts a solid:
The degree of wetting is quantified by the contact angle (θ) formed between the liquid and solid phases at their interface. For effective brazing, the filler metal’s wetting angle should typically be less than 20°.
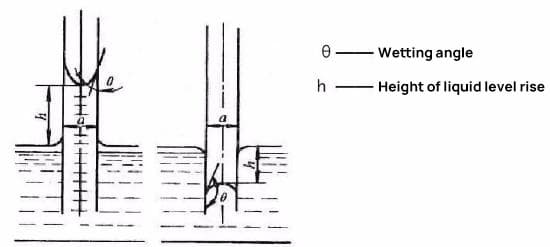
Capillary action:
It is assumed that when two metal plates that are parallel to each other are inserted vertically into an infinite amount of liquid solder, the plates are infinite and the amount of solder is unlimited.
Depending on the wetting properties of the solder on the metal plates, the capillary effect will result in either the situation shown in Figure (a) or the situation shown in Figure (b). If the solder is able to wet the metal plates, the outcome depicted in Figure (a) will occur; if not, the outcome in Figure (b) will occur.
Sn-based and Pb-based Soft Solders:
These solders exhibit excellent wetting and spreading capabilities on copper and various other metals, making them the predominant choice in the electronics industry. Sn-based solders, in particular, are gaining prominence due to environmental concerns and regulatory requirements.
Cd-based Solder:
Primarily composed of cadmium-silver alloys, these solders offer superior heat and corrosion resistance. However, their use is increasingly restricted due to the toxicity of cadmium, leading to the development of safer alternatives.
Zn-based Solder:
Zinc-based solders provide a cost-effective option with good mechanical properties and corrosion resistance. They are particularly useful in applications requiring higher melting points than traditional Sn-Pb solders.
Au-based Soft Solder:
Gold-based solders offer exceptional corrosion resistance and are often used in high-reliability electronic and aerospace applications. Their high cost limits widespread use to specialized sectors.
Other Low Melting Point Soft Solders:
Lead-free Solder:
In response to environmental and health concerns, lead-free solders have been developed and are now widely adopted in the electronics industry. Common lead-free formulations include SAC (Tin-Silver-Copper) alloys, which offer a balance of performance, reliability, and cost-effectiveness. These solders typically require slightly higher processing temperatures and may exhibit different wetting characteristics compared to traditional Pb-Sn solders.

Brazing filler metals play a crucial role in joining components with high strength and reliability. Their selection is critical for achieving optimal joint performance in various industrial applications.
The main categories of brazing filler metals include:
Aluminum-based filler metals:
Specifically designed for brazing aluminum and its alloys. They offer excellent wetting and flow characteristics, ensuring strong bonds while maintaining the base material’s corrosion resistance. Commonly used in aerospace, automotive, and HVAC industries.
Silver-based brazing filler metals:
Known for their exceptional versatility and performance across a wide range of metals. These alloys offer low melting points, excellent flow properties, and high joint strength. They are widely used in electrical, medical, and aerospace applications due to their superior conductivity and corrosion resistance.
Copper-based filler metals:
Nickel-based filler metals:
Although not mentioned in the original text, these are important for high-temperature applications. They offer excellent strength and corrosion resistance at elevated temperatures, making them suitable for aerospace, nuclear, and chemical processing industries.
Precious metal-based filler metals (including gold and palladium):
These specialized filler metals offer unique properties such as high corrosion resistance, biocompatibility, and performance in extreme environments. They are used in critical applications in the medical, aerospace, and electronics industries.
See also:
Function and Performance Requirements of Brazing Flux:
1) Eliminate oxide films on metal surfaces, creating optimal conditions for wetting and spreading of the filler metal.
2) Form a protective liquid barrier over both the base metal and filler metal surfaces during the brazing process.
3) Act as an interfacial agent to enhance wetting characteristics and promote filler metal spreading.
Necessity of Oxide Film Removal During Brazing
The presence of oxide films on metal surfaces significantly impedes the wetting and spreading behavior of brazing filler metals, necessitating their removal for successful joint formation. The challenges associated with oxide film removal are directly proportional to:
Effective oxide film removal can be achieved through various techniques:
Brazing flux serves multiple critical functions beyond oxide removal:
Table 1 Formation Rate of Oxide Film in Dry Air
| Metal | 1 minute | 1 hour | 1 day |
| Stainless steel | 10 | 10 | 10 |
| Iron | 20 | 24 | 33 |
| Aluminum | 20 | 80 | 100 |
| Copper | 33 | 50 | 50 |
In brazing operations, the primary neutral gas utilized is argon, with nitrogen serving as an alternative in specific applications.
Argon, an inert noble gas, primarily functions as a protective atmosphere for the workpiece. While it effectively shields the brazing area from atmospheric contaminants, it lacks the ability to directly remove oxide films from metal surfaces.
The removal of certain oxide films during brazing occurs through a combination of mechanisms:
As illustrated in metallurgical data tables, the decomposition temperatures of most metal oxides significantly exceed both the melting points and boiling points of their respective base metals. This thermal relationship leads to the conclusion that oxide decomposition cannot be achieved solely through the heating process inherent in brazing operations.
To effectively manage oxide films during brazing, additional strategies are often employed:
| Oxide | Decomposition temperature (℃) | Oxide | Decomposition temperature (℃) |
| Au2O | 250 | PbO | 2348 |
| Ag2O | 300 | NiO | 2751 |
| Pt2O | 300 | FeO | 3000 |
| CdO | 900 | MnO | 3500 |
| Cu2O | 1835 | ZnO | 3817 |
1. Iron soldering
Features: low temperature
Scope of application:
1. It is applicable to soldering (using tin lead or lead based filler metal) with soldering temperature lower than 300C;
2. Solder flux is required for brazing thin and small parts.

2. Torch brazing, torch soldering
Features: simple, flexible and widely used
Scope of application: generally, neutral flame or slight carbonization flame/general gas torch or special brazing torch (torch can also be used for soft soldering) shall be used to heat the workpiece first:
1. It is applicable to brazing some weldments which are limited by the shape, size and equipment of weldments and cannot be brazed by other methods
2. Automatic flame brazing can be used
3. Weldable steel, stainless steel, hard alloy, cast iron, copper, silver, aluminum, etc. and their alloys
4. Common filler metals include copper zinc, copper phosphorus, silver base, aluminum base and zinc aluminum filler metals

3. Dip brazing, dip soldering
(Salt bath and metal bath, suitable for mass production)

4. Flow soldering, wave soldering, spray soldering
(A variety of metal bath brazing, mainly used for brazing printed circuit boards)

5. Resistance brazing
Extremely fast heating and high productivity.
6. Induction brazing
Fast heating, less oxidation and small brazing.

The brazing production process encompasses several steps including preparation of the workpiece surface prior to brazing, assembly, placement of the filler metal, brazing, post-brazing treatment, and other related processes.
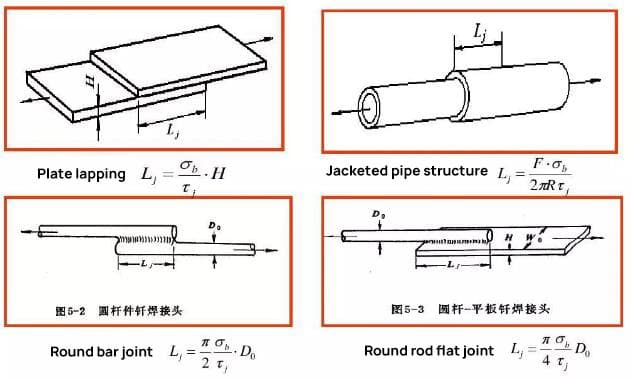
1. Brazed joint design
When designing a brazed joint, the primary consideration should be its strength, followed by the process considerations such as ensuring the dimensional accuracy of the assembly, proper assembly and positioning of the parts, placement of the solder, and the clearance of the brazed joint.
The lap joint is commonly used for brazing joints.
In practical production, for brazing joints made with high-strength silver-based, copper-based, or nickel-based filler metals, the lap length is typically 2-3 times the thickness of the thinner piece.
For soldered joints made with soft solders such as tin-lead, the lap length can be 4-5 times the thickness of the thinner piece, but it should not exceed 15mm.
Types of brazed joints
a) Joint form of plate brazing

b) Joint form of T-shape and bevel brazing

c) Joint form of tube or bar and plate
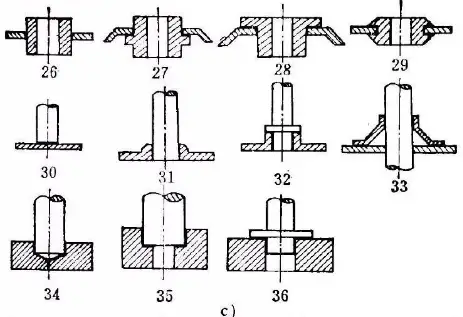
d) Joint form of wire contact brazing

e) Joint form of pipe brazing
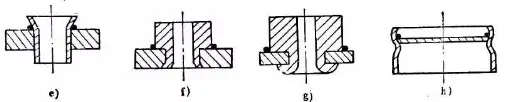
Positioning method of brazed joint

a) Gravity positioning b) Tight fit c) Knurling d) Flanging
e) Flaring f) Spinning g) Die forging h) Necking

i) Undercut j) Slotting and bending k) Clamping l) Positioning pin
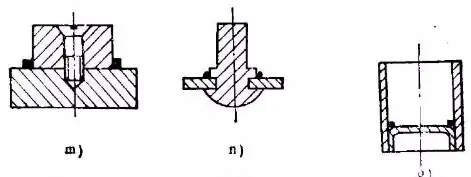
m) Screw n) Riveting o) Spot welding
2. Surface preparation of weldment
Before the brazing process, it is crucial to thoroughly remove any oxide, grease, dirt, and paint from the surface of the workpiece.
In some cases, it may be necessary to pre-coat the parts with a specific metal layer prior to brazing.
(1) Remove oil stain
Oil stains can be removed using organic solvents.
Common organic solvents include alcohol, carbon tetrachloride, gasoline, trichloroethylene, dichloroethane, and trichloroethane.
(2) Oxide removal
Before brazing, the oxide films on the part surface can be processed using mechanical methods, chemical etching methods, and electrochemical etching methods.
3. Assembly and fixing
Solder metals are used in various brazing methods, with the exception of flame brazing and soldering iron brazing, most of which are pre-placed on the joint. The gravity and capillarity of the gap should be utilized as much as possible to encourage the filler metal to fill the gap when placed.
Paste filler metal should be directly applied to the brazed joint, and the powder solder can be mixed with an adhesive before being applied to the joint.
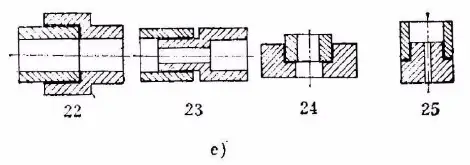
4. Placement method of filler metal
a) Placement of annular solder

b) Placement of foil solder
P – pressure applied