Have you ever wondered why copper and its alloys are so vital in our daily lives? This article explores the fascinating properties of copper, from its incredible electrical conductivity to its impressive corrosion resistance. By the end, you’ll understand why copper remains a cornerstone in modern engineering and technology.

Copper and its alloys are extensively utilized in industrial applications due to their exceptional properties, including superior electrical and thermal conductivity, excellent corrosion resistance, and high formability. These materials are categorized into four main groups: pure copper (often referred to as red copper), brass, bronze, and white copper (also known as nickel silver).
1. Pure Copper: Characterized by its reddish-orange color, pure copper (>99.3% Cu) offers the highest electrical conductivity among commercial metals, making it indispensable in electrical and electronic industries. It also exhibits excellent thermal conductivity, corrosion resistance, and ductility.
2. Brass: An alloy primarily composed of copper and zinc, with zinc content typically ranging from 5% to 45%. Brasses offer a balance of strength, machinability, and corrosion resistance. Common types include:
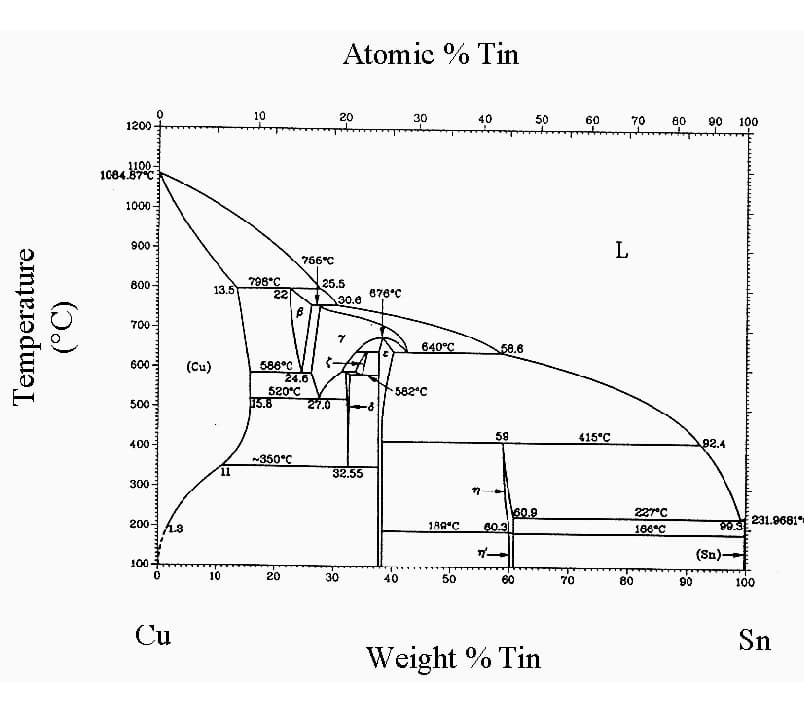
3. Bronze: Traditionally copper-tin alloys, but modern bronzes may contain other elements like aluminum, silicon, or beryllium. Bronzes generally exhibit higher strength and corrosion resistance compared to brasses. Notable types include:
4. White Copper (Nickel Silver): An alloy of copper, nickel, and zinc, typically containing 10-20% nickel. Despite its name, it contains no silver. White copper offers good corrosion resistance, strength, and an attractive silver-like appearance, making it suitable for decorative applications and musical instruments.

Red copper is a pure form of copper with a copper content of at least 99.5%.
It can be further divided into pure copper and oxygen-free copper based on its oxygen content.
Cu2O and CuO oxides can form on the surface of red copper.
At room temperature, the copper surface is covered with Cu2O.
Under high temperatures, the oxide scale is composed of two layers: the outer layer is CuO and the inner layer is Cu2O.
It is important to note that pure copper cannot be brazed in a hydrogen-containing reducing atmosphere.
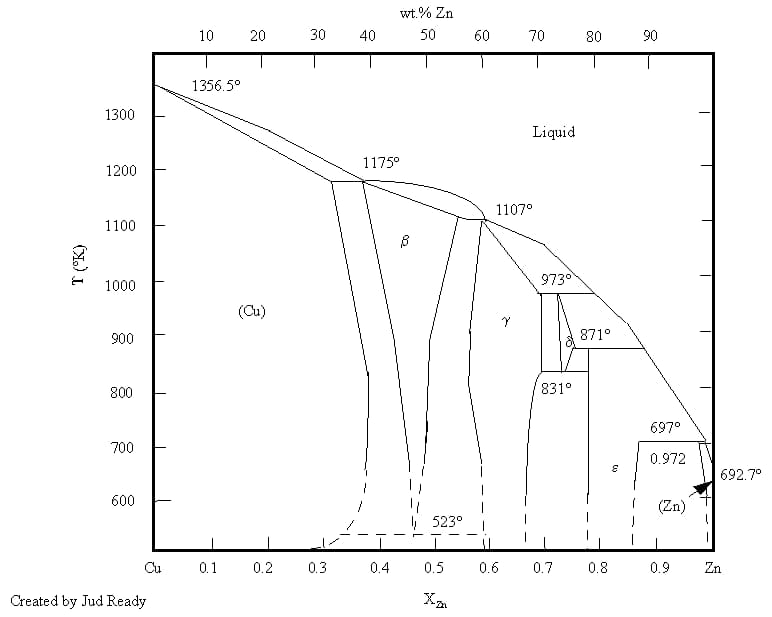
Brass refers to a copper-zinc alloy that has greater strength, hardness, and corrosion resistance compared to red copper while still retaining toughness and high corrosion resistance.

Metallographic diagram of brass
(1) Tin brass:
Tin brass contains approximately 1% tin (Sn) and the presence of tin does not alter the composition of surface oxides.
The solderability of tin brass is comparable to that of brass, making it easy to solder.
(2) Lead brass:
Lead brass contains lead, which when heated forms a sticky slag that impairs the wetting effect and fluidity of solder.
It is important to select the proper flux to ensure proper fluidity.
(3) Manganese brass:
The surface of manganese brass is comprised of zinc oxide and manganese oxide.
Manganese oxide is relatively stable and difficult to remove, so it is necessary to use an active brazing flux to ensure the wettability of the brazing filler metal.
There are various types of bronze, each with different alloy elements, which affects their brazability.
When the alloy element added is tin, or a small amount of chromium or cadmium, it has minimal impact on solderability and is generally easier to braze.
However, if the added element is aluminum, particularly when the aluminum content is high (up to 10%), the aluminum oxide on the surface is difficult to remove, causing a deterioration in solderability.
In such cases, it is necessary to use a special flux for brazing.
For instance, when silicon is added to form silicon bronze, it becomes highly sensitive to hot brittleness and stress cracking when exposed to molten solder.
Another example is when the added alloy element is beryllium.
Although a relatively stable BeO oxide forms, conventional brazing flux is sufficient for removing the oxide film.
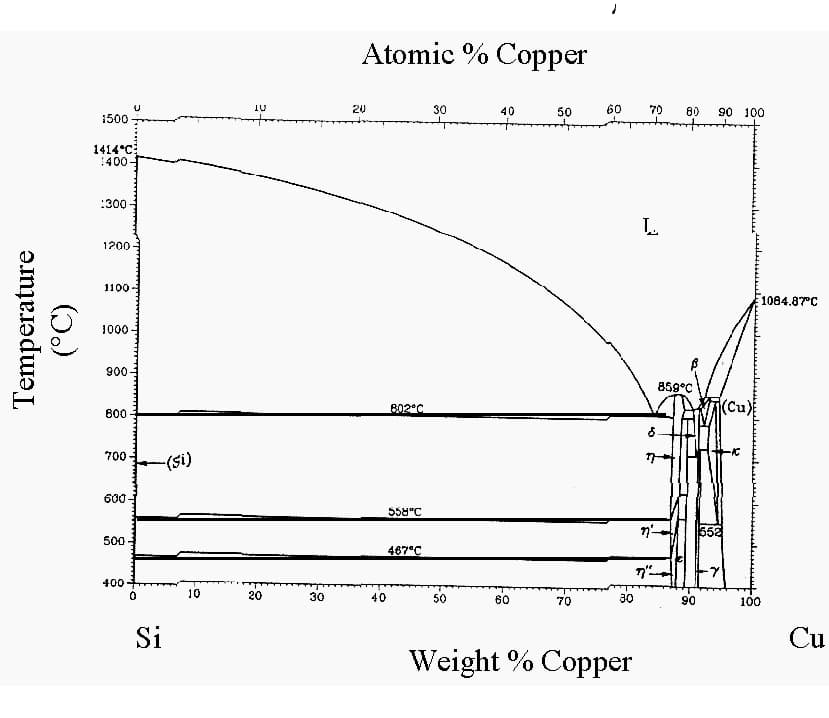
White copper is an alloy of copper and nickel that boasts excellent comprehensive mechanical properties.
It contains nickel.
When selecting filler metal, it is important to avoid those containing phosphorus, such as copper-phosphorus filler metal and copper-phosphorus-silver filler metal.
White copper is highly sensitive to hot cracking and stress cracking when subjected to molten solder.
| Name | Code | Primary Chemical Composition (Mass Percentage, %) | Melting Temperature/℃ | Heat Treatment | |||||||
| ω(Cu) | ω(Zn) | ω(Sn) | ω(Pb) | ω(Mn) | ω(Al) | ω(Ni) | Others | ||||
| Pure Copper | T1 | ≤99.95 | — | — | — | — | — | — | 20.02 | 1083 | Annealing: 450~520℃ |
| T2 | ≤99.90 | — | — | — | — | — | — | 20.06 | 1083 | Annealing: 500~630℃ | |
| Oxygen-Free Copper | TU1 | ≤99.97 | — | — | — | — | — | — | 20.003 | 1083 | Vacuum Annealing: 500℃ |
| TU2 | ≤99.95 | — | — | — | — | — | — | 20.003 | 1083 | ||
| TUMn | ≤99.60 | — | — | — | 0.1~0.3 | — | — | 20.003 | 1083 | ||
| Brass | H96 | 95~97 | Rem. | — | — | — | — | — | — | 1056~1071 | Annealing: 600℃ |
| H68 | 67~70 | Rem. | — | — | — | — | — | — | 910~939 | Annealing: 600℃ | |
| H62 | 60.5~63.5 | Rem. | — | — | — | — | — | — | 899~906 | Annealing: 600℃ | |
| Tin Brass | HSn62-1 | 61~63 | Rem. | 0.7~1.1 | — | — | — | — | — | 886~907 | Annealing: 600℃ |
| Lead Brass | HPb59-1 | 57~60 | Rem. | — | 0.8~1.9 | — | — | — | — | 886~901 | Annealing: 600℃ |
| Manganese Brass | HMn58-2 | 57~60 | Rem. | — | — | 1~2 | — | — | — | 866~881 | Annealing: 600℃ |
| Tin Bronze | QSn6.5-0.1 | Rem. | — | 6~7 | — | — | — | — | P: 0.1~0.25 | ~996 | Annealing: 500~620℃ |
| QSn4-3 | Rem. | 2.7~3.3 | 3.5~4.5 | — | — | — | — | — | ~1046 | ||
| Aluminum Bronze | QAl9-2 | Rem. | — | — | — | 1.5~2.5 | 8~10 | — | — | ~1061 | Annealing: 700~750℃;Quenching880℃,Tempering400℃ |
| QAl10-4-4 | Rem. | — | — | — | — | 9.5~11 | — | Fe: 3.5~4.5 | — | Annealing: 700~750℃;Quenching920℃,Tempering650℃ | |
| Beryllium Bronze | QBe2 | Rem. | — | — | — | — | — | 0.2~0.5 | Be: 1.9~2.2 | 865~956 | Quenching: 800℃,Aging:300℃ |
| QBe1.7 | Rem. | — | — | — | — | — | 0.2~0.4 | Be: 1.6~1.8 | — | Quenching: 800℃,Aging: 300℃ | |
| Silicon Bronze | QSi3-1 | Rem. | — | — | — | 1~1.5 | — | — | Si: 2.75~3.5 | 971~1026 | Annealing: 600~680℃ |
| Chromium Bronze | QCr0.5 | Rem. | — | — | — | — | — | — | Cr: 0.5~1.0 | 1073~1080 | Quenching: 950~1000℃ |
| Aging: 400~460℃ | |||||||||||
| Cadmium Bronze | QCd1 | Rem. | — | — | — | — | — | — | Cd: 0.9~1.2 | 1040~1076 | Annealing: 650℃ |
| Zinc Nickel Silver | BZn15-20 | Rem. | 18~20 | — | — | — | — | 13.5~16.5 | — | ~1081 | Annealing: 700℃ |
| Manganese Nickel Silver | BMn40-1.5 | Rem. | — | — | — | 1~2 | — | 39~40 | — | 1261 | Annealing: 1050~1150℃ |
The brazing of copper and copper alloys primarily depends on the following factors:
Pure copper surfaces may form two oxides, Cu2O and CuO. At room temperature, a copper surface is covered by Cu2O, while at high temperatures, the oxide film is split into two layers, with CuO on the outside and Cu2O on the inside. Copper oxides are easy to remove, so pure copper brazes well.
Oxygenated copper is refined copper using pyrometallurgy and electrolytically tough pitch copper. It contains 0.02% to 0.1% oxygen by mass, which exists as copper oxide, forming a eutectic organization with the copper. This eutectic organization is distributed in the copper matrix in globular form.
If oxygenated copper is brazed in a hydrogen-containing reducing atmosphere, hydrogen rapidly diffuses into the metal, reducing the oxide to produce steam. This steam forms cavities within the copper crystals and expands rapidly, leading to hydrogen embrittlement. In severe cases, the copper material may fracture.
If the atmosphere contains carbon monoxide and moisture, the carbon monoxide can reduce the steam to hydrogen, which then diffuses into the metal, resulting in hydrogen embrittlement. Therefore, oxygenated copper should not be brazed in decomposing ammonia, endothermic, or exothermic reducing atmospheres.
Long-term heating of oxygenated copper above 920℃ will cause copper oxide to accumulate at grain boundaries, lowering the copper’s strength and ductility. Therefore, during brazing, the material should avoid prolonged exposure to temperatures above 920℃.
Copper cannot be heat-treated for strength, so cold working methods are often used to increase its strength. Cold-work-hardened copper will soften when heated between 230℃ and 815℃. The degree of softening depends on the temperature and the duration at this temperature. The higher the brazing heating temperature, the softer the cold-work-hardened copper becomes.
Oxygen-free copper has a low oxygen content, and there are no eutectic constituents of copper and copper oxide in the copper. Its electrical conductivity and cold workability (such as deep drawing and spinning) are better than those of deoxidized copper.
Oxygen-free copper can be brazed in a hydrogen-containing protective atmosphere without hydrogen embrittlement. Cold-work-hardened oxygen-free copper will also soften during heating.
Common brass can be divided into three categories: low brass (zinc mass fraction less than 20%), high brass (zinc fraction more than 20%), and alloy brass. When the mass fraction of zinc in brass is less than 15%, the surface oxide mainly consists of Cu2O, which contains small particles of ZnO.
When the mass fraction of zinc is more than 20%, the oxide primarily comprises ZnO. Zinc oxide is also easy to remove, so the brazing of brass is very good. Brass is not suitable for brazing in a protective atmosphere, especially not in vacuum brazing. This is because zinc has a high vapor pressure (reaching 105Pa at 907℃).
During brazing in a protective atmosphere, especially vacuum brazing, the zinc in the brass volatilizes, the surface turns red, and it affects both its brazing and inherent properties. If brazing must be done in a protective atmosphere or in a vacuum, a layer of copper or nickel should be electroplated on the surface of the brass parts beforehand to prevent zinc volatilization. However, the plating may affect the strength of the brazed joint.
Brazing brass requires the use of a flux.
Tin brass has approximately 1% of ω (Sn). The presence of tin does not affect the composition of the surface oxide. The brazing of tin brass is comparable to that of brass and is easy to braze.
Lead brass forms a sticky residue when heated, which disrupts the wetting action and fluidity of the brazing material, so an appropriate flux must be chosen to ensure the wetting action of the brazing material. When lead brass is heated, it tends to stress crack. Its sensitivity to hot cracking is directly proportional to the lead content.
Therefore, the internal stress of lead brass should be minimized during brazing, such as by annealing before welding to remove the stress caused by component processing. The heating temperature should be as uniform as possible to reduce thermal stress. The brazing effect is poor when ω (Pb) > 3%. For lead brass with ω (Pb) > 5%, brazing is not recommended.
The surface of manganese brass is composed of zinc oxide and manganese oxide. Manganese oxide is relatively stable and difficult to remove, so a highly active flux should be used to ensure the wettability of the brazing material.
Tin bronze QSn6.5-0.1 forms two oxides on its surface: an inner layer of SnO2 and an outer layer of copper oxide. These oxides are easy to remove, and the alloy brazes well, suitable for various brazing methods including gas shielded brazing and vacuum brazing.
Conventional fluxes can be used for brazing in air. To avoid cracking, phosphorous-containing tin bronze parts should be stress relieved at approximately 290-340℃ before brazing.
Aluminum bronze contains a significant amount of aluminum (up to 10% by mass), forming an oxide layer mainly composed of aluminum oxide on the surface, which is difficult to remove. Therefore, brazing aluminum bronze is quite challenging. Aluminum oxide cannot be reduced in a protective atmosphere and cannot be removed by vacuum heating, requiring a specialized flux.
If aluminum bronze parts are brazed in a quenched and tempered state, the brazing temperature should not exceed the tempering temperature. For example, the tempering temperature of QAl9-2 is 400℃.
If the brazing temperature exceeds 400℃, the base material will soften. If brazing is done at high temperatures, the brazing temperature should match the quenching temperature (880℃), followed by tempering, to achieve the desired mechanical properties of the base material. This must be considered when selecting a brazing material.
Although a relatively stable BeO oxide forms on the surface of beryllium bronze, conventional flux still satisfies the requirement of oxide film removal. Beryllium bronze is often used in situations where parts require elasticity.
To avoid lowering this property, the brazing temperature should be either below its aging temperature (300℃) or the brazing temperature should match the quenching temperature, followed by aging treatment after brazing.
Silicon bronze, mainly QSi3-1 alloy with about 3% ω (Si), forms an oxide primarily composed of silicon dioxide on its surface. The same flux used for brazing aluminum bronze should be used for brazing silicon bronze. Silicon bronze under stress is extremely sensitive to thermal cracking and stress cracking under the action of molten brazing material.
To avoid cracking, the alloy should be stress relieved at a temperature between 300-350℃ before brazing. A lower melting point brazing material should be chosen and a brazing method that evenly heats should be used during brazing.
Chromium bronze and cadmium bronze have minor amounts of chromium or cadmium, which do not significantly affect the brazing process. When brazing chromium bronze, the heat treatment regime of the base material should be considered.
Brazing should either occur below the aging temperature (460℃) or the brazing temperature should match the quenching temperature (950-1000℃).
Nickel silver and manganese silver. Nickel silver contains nickel, and phosphorus-containing brazing materials, such as copper-phosphorus brazing material and copper-phosphorus-silver brazing material, should be avoided when choosing a brazing material because phosphorus-containing brazing materials can easily form brittle nickel phosphide on the interface after brazing, reducing the strength and toughness of the joint.
Nickel silver is extremely sensitive to both hot cracking and stress cracking under the action of molten brazing material. Therefore, parts should have their internal stress removed before brazing, and a lower melting point brazing material should be chosen.
Parts should be heated evenly, and free expansion and contraction of the parts during heating and cooling should be allowed to reduce thermal stress during brazing.
Brazability of common copper and copper alloys
| Alloy | Brazeability | |
| Copper T1 | Excellent | |
| Oxygen-free copper TU1 | Excellent | |
| Brass | H96 | Excellent |
| H68 | Excellent | |
| H62 | Excellent | |
| Tin-bronze | HSn62-1 | Excellent |
| Manganese brass | HMn58-2 | Good |
| Tin-bronze | QSn58-2 | Excellent |
| QSn4-3 | Excellent | |
| Lead Brass | HPb59-1 | Good |
| aluminium bronze | QAl9-2 | Bad |
| QAl10-4-4 | Bad | |
| beryllium bronze | QBe2 | Good |
| QBe1.7 | Good | |
| silicon bronze | QSi3-1 | Good |
| chromium bronze | QCr0.5 | Good |
| cadmium bronze | QCd11 | Excellent |
| Zinc-Copper-nickel alloy | BZn15-20 | Good |
| Mn copper nickel alloy | BMn40-1.5 | Difficult |
Silver-based solder is widely utilized due to its moderate melting point, good processability, strong and tough qualities, conductivity, thermal conductivity, and corrosion resistance.
The main alloy elements of silver-based solders are copper, zinc, cadmium, and tin. Copper is the most important alloy element, as it reduces the melting temperature of silver without forming a brittle phase.
The addition of zinc further lowers the melting temperature.
While the addition of tin can significantly lower the melting temperature of silver-copper-tin alloys, this low melting temperature results in extreme brittleness and lack of practical use.
To avoid brittleness, the tin content in silver-copper-tin solder is typically not higher than 10%.
To further reduce the melting temperature of silver-based solder, cadmium can be added to the silver-copper-zinc alloy.
Chemical composition and main properties of silver based brazing filler metal
| Brazing filler metal | Chemical composition(weight %) | Melting temperature/℃ | Tensile strength/MPa | Electrical resistivity/μΩ·m | Brazing temperature/℃ | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ag | Cu | Zn | Cd | Sn | other | |||||
| BAg72Cu. | 72±1 | Rem. | – | – | – | – | 779~779 | 375 | 0.022 | 780~900 |
| BAg50Cu. | 50±1.1 | Rem. | – | – | – | – | 779~850 | – | – | – |
| BAg70Cu. | 70±1 | 26±1 | Rem. | – | – | – | 730~755 | 353 | 0.042 | – |
| BAg65Cu. | 65±1 | 20±1.1 | Rem. | – | – | – | 685~720 | 384 | 0.086 | – |
| BAg60Cu | 60 ±1 | Rem. | – | 10±0.5 | – | – | 602~718 | – | 720~840 | |
| BAg50Cu | 50±1.1 | 34±1.1 | Rem. | – | 10±0.5 | – | 677~775 | 343 | 0.076 | 775~870 |
| BAg45Cu | 45±1 | 30+1 | Rem. | – | – | – | 677~743 | 386 | 0.097 | 745~845 |
| BAg25CuZn. | 25±1. | 40±1 | Rem. | – | – | – | 745~775 | 353 | 0.069 | 800~890 |
| BAg10CuZn | 10±1 | 53±1.1 | Rem. | – | – | – | 815~850 | 451 | 0.065 | 850~950 |
| BAg50CuZnCd | 50±1 | 15.5±1 | 16.5±2 | – | – | – | 627~635 | 419 | 0.072 | 635~760 |
| BAg45CuZnCd | 45±1. | 15±1 | 16±2. | – | – | – | 607~618 | – | – | 620~760 |
| BAg40CuZnCdNi | 40±1 | 16±0.5 | 17.8±0.5 | – | – | Ni0.2±0.1 | 595~605 | 392 | 0.069 | 605~705 |
| BAg34CuZnCd | 35±1 | 26±1 | 21±2 | – | – | 607~702 | 411 | 0.069 | 700~845 | |
| BAg50CuZnCdNi | 50±1.1 | 15.5±1 | 15.5±2 | – | – | Ni3±0.5 | 632~688 | 431 | 0.105 | 690~815 |
| BAg56CuZnSn | 56±1 | 22±1 | 17±2 | 50.5 | 50.5 | – | 618~652 | – | – | 650~760 |
| BAg34CuZnSn | 34±1 | 36±1.1 | 27+2 | 30.5 | 30.5 | – | 630~730 | – | – | 730~820 |
| BAg50CuZnSnNi | 50±1 | 21.5±1 | 27±1.1 | 10.3 | 10.3 | Ni0.30~0.65。 | 650~670 | – | – | 670~770 |
| BAg40CuZnSnNi | 40±1 | 25±1 | 30.5±1 | 30.3 | 30.3 | Ni1.30~1.65 | 630~640. | – | – | 640~740 |
Copper-phosphorus brazing filler metal is widely utilized in brazing copper and copper alloys due to its favorable technological performance and cost-effectiveness.
Phosphorus serves two functions in copper:
First, it significantly lowers the melting point of copper.
Second, it acts as a self-soldering flux during brazing in air.
To further reduce the melting temperature of the Cu-P alloy and improve its toughness, silver can also be added.
It is important to note that copper-phosphorus and copper-rattan-silver filler metals can only be used for brazing copper and copper alloys and cannot be used for brazing steel, nickel alloys, or copper-nickel alloys with a nickel content greater than 10%.
This type of filler metal may result in segregation when heated slowly, so it is best to adopt a fast heating brazing method.
Chemical composition and properties of copper phosphorus solder
| Filler metal | Chemical composition (mass fraction) (%) | Melting temperature | Tensile strength MPa | Resistivity/μΩ·m | ||||
|---|---|---|---|---|---|---|---|---|
| Cu | P | Ag | Sn | other | ||||
| Bcu95P. | Rem. | 5±0.3 | – | – | 710~924 | – | – | |
| Bcu93P | Rem. | 6.8~7.5 | – | – | 710~800 | 470.4 | 0.28 | |
| Bcu92PSb | Rem. | 6.3±0.4 | – | – | Sb1.5~2.0 | 690~800 | 303.8 | 0.47 |
| Bcu91Ag | Rem. | 7±0.2 | 2±0.2 | – | – | 645~810 | – | – |
| Bcu89Ag | Rem. | 5.8~6.7 | 5±0.2 | – | – | 650~800 | 519.4 | 0.23 |
| Bcu80Pag | Rem. | 4.8~5.3 | 15±0.5 | – | – | 640~815 | 499.8 | 0.12 |
| HLAgCu70-5 | Rem. | 5±0.5 | 25±0.5 | – | – | 650~710 | – | – |
| HLCuP6-3 | Rem. | 5.7±0.3 | – | 3.5±0.5 | – | 640~680 | – | 0.35 |
| Cu86SnP | Rem. | 5.3±0.5 | – | 7.5±0.5 | 0.8±0.4 | 620~660 | – | – |
| Bcu80PSnAg | Rem. | 5.3±0.5 | 5±0.5 | 10±0.5 | – | 560~650 | – | – |
| Cu77NiSnP. | 77.6 | 7.0 | 9.7 | – | Ni5.7 | 591~643 | – | – |
When brazing copper with Sn-based solder, the formation of the intermetallic compound Cu6Sn5 at the interface between the solder and base metal is common. Therefore, it is important to carefully consider the brazing temperature and holding time.
When using a soldering iron, the compound layer is typically thin and has minimal impact on the joint’s performance.
Brass joints brazed with tin-lead filler metal are stronger than copper joints brazed with the same filler metal. This is because the dissolution of brass in the liquid filler metal is slower, resulting in the formation of fewer brittle intermetallic compounds.
| Brazing filler metal | Chemical composition | Fusion temperature | Tensile strength | Elongation | |||
|---|---|---|---|---|---|---|---|
| Sn | Ag | Sb | Cu | ||||
| HL606 | 96.0 | 4.0 | – | – | 221 | 53.0 | – |
| Sn95Sb | 95.0 | – | 5.0 | – | 233 | 39.2 | 43 |
| Sn92AgCuSb | 92.0 | 5.0 | 1.0 | 2.0 | 250 | 49.0 | 2.3 |
| Sn85AgSb | 84.5 | 8.0 | 7.5 | – | 270 | 80.4 | 8.8 |
| Brazing filler metal | Chemical composition | Fusion temperature | ||
|---|---|---|---|---|
| 97.0 | 3.0 | Sn | ||
| HLAgPb97 | 97.5 | 1.5 | – | 304-305 |
| HLAgPb97.5-1.0 | 92 | 2.5 | 1.0 | 310-310 |
| HLAgPb92-5.5 | 83.5 | 1.5 | 5.5 | 287-296 |
| HLAgPb83.5-15-1.5 | 97.0 | 3.0 | 15.0 | 265-270 |
Chemical composition and properties of cadmium based solder
| Filler metal | Chemical composition (mass fraction) (%) | Melting temperature/ | Tensile strength/MPa | ||
|---|---|---|---|---|---|
| Cd | Ag | Zn | |||
| HL503 | 95 | 5 | 338~393 | 112.8 | |
| HLAgCd96-1 | 96 | 3 | 1 | 300~325 | 110.8 |
| Cd79ZnAg | 79 | 5 | 16 | 270~285 | 200 |
| HL508 | 92 | 5 | 3 | 320~360 | – |
Lead free solder for brazing copper tubes
| Brand | Composition (mass fraction) | Solid phase line/℃ | Liquidus/℃ |
| E | 95Sn-4.5Cu-0.5Ag | 226 | 360 |
| HA | 94.5Sn-3Sb-1.5Zn-0.5Ag-0.5Cu | 215 | 228 |
| HB | 91.225Sn-5Sb-3.5Cu-0.275Ag | 238 | 360 |
| AC | 96.25n-3.25Bi-0.2Cu-0.35Ag | 206 | 234 |
| OA | 95.9Sn-3Cu-1Bi-0.1Ag | 215 | 238 |
| AM | 95.45n-3Cu-1Sb-0.6Ag | 221 | 231 |
Strength of Copper and Brass Joints Brazed with Part of Soft Solder
| Solder brand | Shear strength/MPa | Tensile strength/MPa | ||
|---|---|---|---|---|
| copper | brass | copper | brass | |
| S-Pb80Sn18Sb2 | 20.6 | 36.3 | 88.2 | 95.1 |
| S-Pb68Sn30Sb2 | 26.5 | 2740 | 89.2 | 86.2 |
| S-Pb58Sn40Sb2 | 36.3 | 45.1 | 76.4 | 78.40 |
| S-Sn90Pb10 | 45.1 | 44.1 | 63.7 | 68.6 |
| S-P697Ag3 | – | 29.4 | – | 49.0 |
| S-Cd96Ag3Zn1 | 73.5 | – | 57.8 | — |
| S-Sn95Sb5 | 37.2 | – | — | |
| S-sn85Ag8Sb7 | – | 82.3 | – | – |
| S-Sn92AgSCu2Sb1 | 35.3 | – | – | – |
| S-Sn96Ag4P | 35.339.2~49.0 | – | 5.339.2~49.0 | – |
The commonly used brazing fluxes consist of a matrix of borax, boric acid, or a mixture of both, and are supplemented with fluorides or fluoroborates of alkali or alkaline earth metals to achieve an appropriate activation temperature and improve oxide removal capabilities.
When heated, boric acid (H3BO3) breaks down to form boric anhydride (B2O3).
The reaction formula is as follows:
2H3BO3→B2O3+3H2O
The melting point of boric anhydride is 580°C.
It can react with copper, zinc, nickel, and iron oxides to form a soluble borate, which floats on the brazed joint as a slag. This not only removes the oxide film but also provides mechanical protection.
MeO+B2O3→MeO-B2O3
Borax Na2B4O7 melts at 741 ℃:
Na2B4O7→B2O3+2NaBO2
Boric anhydride and metal oxides react to form soluble borates. Sodium metaborate and borates combine to form compounds with a lower melting temperature, making them easy to rise to the surface of solder joints.
MeO+2NaBO2+B2O3>(NaBO2)2Me(BO2)2
The combination of borax and boric acid is a commonly utilized flux. The addition of boric acid can lower the surface tension of the borax flux and enhance its spread. Boric acid also enhances the ability of the flux residue to cleanly detach from the surface. However, when using borax-boric acid flux with silver filler metal, its melting temperature remains too high and its viscosity is still too high.
To further decrease the melting temperature, potassium fluoride can be added. The primary role of potassium fluoride is to lower the viscosity of the flux and enhance its ability to remove oxides. To further reduce the melting temperature and increase its activity, KBF4 can be added.
The melting point of KBF4 is 540 ℃, and the melting decomposition is:
KBF4→KF+BF3
| Brand | Composition (mass fraction) (%) | Action temperature ℃ | Purpose |
| FB101 | Boric acid 30, potassium fluoroborate 70 | 550~850℃ | Silver solder flux |
| FB102 | Anhydrous potassium fluoride 42, potassium fluoroborate 25, boric anhydride 35 | 600~850℃ | The most widely used silver solder flux |
| FB103 | Potassium fluoborate>95, potassium carbonate<5 | 550~750℃ | For silver copper zinc cadmium solder |
| FB104 | Borax 50, boric acid 35, potassium fluoride 15 | 650~850℃ | Brazing with silver based filler metal in furnace |
| Number | Component | Purpose |
| 1 | ZnCl21130g,NH4Cl110g,H2O4L | Brazing copper and copper alloys, steel |
| 2 | ZnCl21020g,NaCI280g,NH4CI,HCI30g,H2O4L | Welding copper and copper alloys, steel |
| 3 | ZnCl2600g,NaCl170g | Dipped brazing covering agent |
| 4 | ZnCl2710g, NH4Cl100g, Vaseline 1840g, H2O 180g | Brazing copper and copper alloys, steel |
| 5 | ZnCl21360g,NH4Cl140g,HC185g,H2O4L | Brazing silicon bronze, aluminum bronze, stainless steel |
| 6 | H3P04960g,H20455g | Brazed manganese bronze, Stainless steel |
| QJ205 | ZnCl250g,NH4Cl15,CdCl230,NaF6 | Brazing of copper and copper alloys with cadmium based filler metals |
| Number | Component | Purpose |
| 1 | Glutamic acid hydrochloride 540g, urea 310g, water 4L | Copper, brass, bronze |
| 2 | Hydrazine monobromide 280g, water 2550g, non-ionic wetting agent 1.5g | Copper, brass, bronze |
| 3 | Lactic acid (85%) 260g, water 1190g, wetting agent 3g | Wrinkled bronze |
The main component of non corrosive flux is rosin.
There are three commonly used rosin fluxes:
Copper and its alloys exhibit excellent brazability due to their high thermal conductivity and favorable wetting characteristics. Various brazing methods can be employed, each offering distinct advantages for specific applications:
When brazing copper and its alloys, special considerations are necessary:
For high-frequency brazing of copper, the process requires careful optimization due to copper’s low electrical resistance. Strategies to overcome this challenge include:
When brazing copper, the coordination of filler metal and flux is as follows:
When soldering clean surfaces, especially with tin lead and tin silver solder, rosin flux can be used. For other surfaces, active rosin, weak corrosive flux, or corrosive flux can be utilized.
It is important to note that pure copper should not be brazed in a reducing atmosphere, except for oxygen-free copper, in order to avoid hydrogen embrittlement.
The filler metal and flux used for brazing brass are generally similar to those used for brazing copper. However, it should be noted that due to the presence of zinc oxide on the surface of brass, it cannot be brazed with inactive rosin. Additionally, when brazing with copper phosphorus and silver solder, FB102 flux must be used.
For tin-lead brazing, a phosphoric acid solution flux should be utilized. Lead-based brazing requires the use of a zinc oxide solution brazing flux. Q205 brazing flux is used for cadmium-based brazing. BAg45CuCdNi and BAg45CuCd solders should be brazed with FB102 or FB103 flux. Other silver-based solders, as well as copper phosphorus and copper phosphorus silver solders, should be brazed with FB102 flux. It is recommended to braze using FB104 flux in a protective atmosphere within a furnace.
When brazing beryllium bronze in its soft soldering quenching aging state, it is important to select a brazing filler metal with a melting temperature lower than 300°C. The preferred combination for this application is 63Sn-37Pb in combination with a weak corrosive flux or a corrosive flux.
Additionally, brazing and solution treatment should be carried out simultaneously during the brazing process.
Soft soldering has minimal impact on the performance index of beryllium bronze, thus soft solders and fluxes similar to those used for beryllium bronze can be utilized for brazing.
It is important to note that chromium bronze should not be brazed in its solution aging state, but rather in the solution treatment state followed by aging.
When using a rapid heating method for brazing, it is recommended to use the silver solder with the lowest melting temperature, such as BAgA0 CuZnCdNi.
Brazing tin bronze is similar to brazing copper and brass, but with the added benefit of avoiding hydrogen embrittlement and zinc volatilization when brazing in a protective atmosphere.
However, it should be noted that tin bronze containing phosphorus has a tendency towards stress cracking.
For soft soldering, it is recommended to use a strong corrosive flux containing hydrochloric acid.
During brazing, there is a tendency towards stress cracking and intergranular penetration of the filler metal. The brazing temperature should be below 760°C.
Silver solders with lower melting temperatures, such as BAg65CuZn, BAg50 CuZnCd, BAg40 CuZnCdNi, and BAg56 CuZnSn, can be used. The lower the melting temperature, the better.
For optimal results, FB102 and FB103 are the recommended fluxes to use.
When performing soft soldering, it is important to use a strong corrosive flux containing hydrochloric acid to remove the oxide film on the surface. The commonly used solder for this process is tin-lead solder.
For brazing, silver filler metal is typically used. To prevent aluminum from diffusing into the silver solder, the brazing heating time should be kept as short as possible. Plating the surface of aluminum bronze with copper or nickel can also prevent the diffusion of aluminum into the solder.
The soldering process for zinc white copper is similar to that of brass. The following silver solders are commonly used for brazing: BAg56CuZnSn, BAg50CuZnSnNi, BAg40CuZnNi, and BAg56CuZnCd, among others. The recommended fluxes for use are FB102 and FB103.
For soldering zinc white copper, a phosphoric acid solution flux can be used or the surface can be pre-plated with copper.
The brazing filler metals that can be used include BAg60CuZn, BAg45CuZn, BAg40CuZnCdNi, and BAg50 CuZnCd, among others.
It is not recommended to use copper-phosphorus silver solder, as phosphorus and nickel will form a brittle compound phase.
Joint Strength of Copper and Brass Brazed with Silver Solder
| Filler metal | Shear strength/MPa | Tensile strength/MPa | ||
|---|---|---|---|---|
| copper | brass | copper | brass | |
| BAg45CuZn | 177 | 215 | 181 | 325 |
| BAg50CuZn | 171 | 208 | 174. | 334 |
| BAg65CuZn | 171 | 208 | 177 | 334 |
| BAg70CuZn | 166 | 199 | 185 | 321 |
| BAg40CuZnCdNi | 167 | 194 | 179 | 339 |
| BAg50CuZnCd | 167 | 226 | 210 | 375 |
| BAg35CuZnCd | 164 | 190 | 167 | 328 |
| BAg40CuZnSnNi | 98 | 245 | 176 | 295 |
| BAg50CuZnSn | – | – | 220 | 240 |
Mechanical properties of copper joints brazed with copper phosphorus and copper phosphorus silver solders
| Filler metal | Tensile strength /MPa | Shear strength /MPa | Bending angle (°) | Impact toughness /J · cm-2 |
| BCu93P | 186 | 132 | 25 | 6 |
| BCu92PSb | 233 | 138 | 90 | 7 |
| BCu80PAg | 255 | 154 | 120 | 23 |
| BCu89PAg | 242 | 140 | 120 | 21 |
For age-hardenable copper alloys, such as beryllium bronze, that have undergone heat treatment, the only step after brazing is to remove the residual flux and clean the workpiece surface.
The primary reason for removing the residue is to prevent corrosion on the workpiece and, in some cases, to achieve a good appearance or prepare the workpiece for further processing.
The joint strength of copper and brass soft brazed joints using several commonly used soft brazing materials is shown in Table 10.
Table 10: Joint Strength of Copper and Brass Soft Brazed Joints
| Brazing material grade | Shear Strength /MPa | Tensile Strength /MPa | ||
| Copper | Brass | Copper | Brass | |
| S-Pb80Sn18Sb2 | 20.6 | 36.3 | 88.2 | 95.1 |
| S- Pb68Sn30Sb2 | 26.5 | 27.4 | 89.2 | 86.2 |
| S-Pb58Sn405b2 | 36.3 | 45.1 | 76.4 | 78.4 |
| S-Pb97Ag3 | 33.3 | 34.3 | 50.0 | 58.8 |
| S-Sn90Pb10 | 45.1 | 44.1 | 63.7 | 68.6 |
| S-Sn95Sb5 | 37.2 | – | – | – |
| S-Sn92Ag5Cu2Sb1 | 35.3 | – | – | – |
| S-Sn85Ag85B7 | 一 | 42.3 | – | – |
| S-Cd96Ag3Znl | 57.8 | – | 73.8 | – |
| S-Cd95Ag5 | 44.1 | 46.0 | 87.2 | 88.2 |
| S-Cd92Ag5Zn3 | 48.0 | 54.9 | 90.1 | 96.0 |
When brazing copper with tin-lead solder, non-corrosive fluxes such as rosin alcohol solution or a mixture of activated rosin and ZnCl2 + NH4Cl water solution can be used. The latter can also be used for brazing brass, bronze, and beryllium bronze.
When brazing aluminum brass, aluminum bronze, and silicon brass, a flux consisting of zinc chloride in hydrochloric acid solution can be used. For brazing manganese bronze, a phosphoric acid solution can be used as the flux.
When using lead-based solder, a zinc chloride water solution can be used as the flux, and for cadmium-based solder, FS205 flux can be used.
Hard Brazing Materials and Fluxes for Hard Brazing
When brazing copper, silver-based solder and copper-phosphorus solder can be used. Silver-based solder has a moderate melting point, good processability, and excellent mechanical, electrical, and thermal conductivity properties. It is the most widely used hard brazing material.
For applications requiring high electrical conductivity, a silver-containing solder such as B-Ag70CuZn should be chosen. For vacuum brazing or brazing in a protective atmosphere furnace, silver-based solders without volatile elements, such as B-Ag50Cu and B-Ag60CuSn, should be used.
Solders with lower silver content are cheaper but have higher brazing temperatures and lower joint toughness, making them suitable for brazing applications with lower requirements on copper and copper alloys.
Copper-phosphorus and copper-phosphorus-silver solders can only be used for hard brazing of copper and its alloys. B-Cu93P solder has excellent flowability and is suitable for brazing parts in the mechanical, electrical, instrumentation, and manufacturing industries that are not subjected to impact loads.
The ideal gap size is 0.003-0.005mm. Copper-phosphorus-silver solders (such as B-Cu70Pag) have better toughness and electrical conductivity than copper-phosphorus solder and are mainly used for high-conductivity electrical joints. The performance of several commonly used hard brazing materials for hard brazing of copper and brass joints is shown in Table 11.
Table 11: Performance of Copper and Brass Hard Brazed Joints
| Brazing material grade | Shear Strength /MPa | Tensile Strength /MPa | Bending angle /(°) | Impact absorption energy /J | ||
| Copper | Brass | Copper | Brass | Copper | Copper | |
| H62 | 165 | – | 176 | – | 120 | 353 |
| B-Cu60ZnSn-R | 167 | – | 181 | – | 120 | 360 |
| B-Cu54Zn | 162 | – | 172 | – | 90 | 240 |
| B-Zn52Cu | 154 | – | 167 | – | 60 | 211 |
| B-Zn64Cu | 132 | – | 147 | – | 30 | 172 |
| B-Cu93P | 132 | – | 162 | 176 | – | 58 |
| B-Cu92PSb | 138 | – | 160 | 196 | 25 | – |
| B-Cu93Pag | 159 | 219 | 225 | 292 | – | – |
| B-Cu80Pag | 162 | 220 | 225 | 343 | 120 | 205 |
| B-Cu90P6Sn4 | 152 | 205 | 202 | 255 | 120 | 182 |
| B-Ag70CuZn | 167 | 199 | 185 | 321 | 90 | – |
| B-Ag65CuZn | 172 | 211 | 177 | 334 | – | – |
| B-Ag55CuZn | 172 | 208 | 174 | 328 | – | – |
| B-Ag45CuZn | 177 | 216 | 181 | 325 | – | – |
| B-Ag25CuZn | 167 | 184 | 174 | 316 | – | – |
| B-Ag10CuZn | 158 | 161 | 167 | 314 | – | – |
| B-Ag72Cu | 165 | – | 177 | – | – | – |
| B-Ag50CuZnCd | 177 | 226 | 210 | 375 | – | – |
| B-Ag40CuZnCd | 168 | 194 | 179 | 339 | – | – |