Why do stainless steel pipes, celebrated for their durability, sometimes fail under corrosive conditions? This article dives into the root causes of corrosion cracking in stainless steel pipes, focusing on factors like chemical composition and environmental conditions. Readers will learn about the mechanisms behind these failures and discover effective preventive measures to ensure the longevity of stainless steel piping systems.

Thanks to its outstanding corrosion resistance, 304 stainless steel finds extensive application in equipment and parts that demand good comprehensive properties, such as formability and corrosion resistance. It is extensively employed in various industries, including chemical equipment, pressure vessels, among others.
Related reading: Stainless Steel Grades
The sulfuric acid pipe in a fertilizer plant connects the outlet (0.82 MPa) of the sulfuric acid pump to the reactor.
The pump has a flow rate of 14 m3/h, a head of 63 m, a sulfuric acid concentration of 93.5%, and operates at normal temperature.
The pipeline was replaced in 2016, but after two years of use, there was liquid leakage at the weld of the discharge pipe at the inlet and outlet of the pump, as well as at the high-neck flange at the pressure gauge interface.
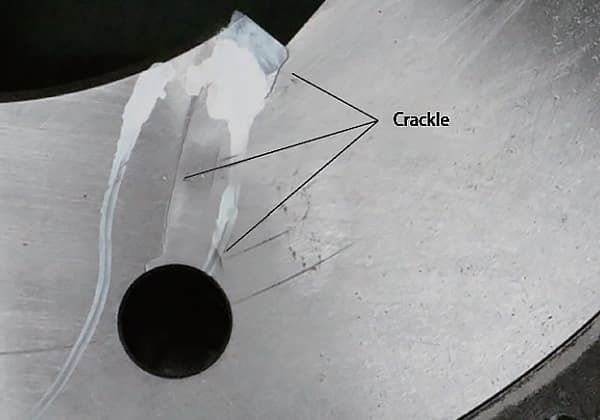
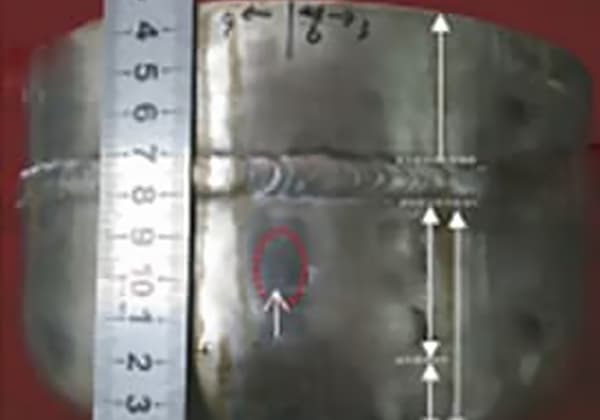
Upon cleaning and penetrant testing of the pipe wall, cracks were discovered (refer to Fig. 1).
Based on the original data, the steel pipe is made of 304 stainless steel, has a diameter of DN50, and a wall thickness of 3.5 mm.
After welding, the penetration test was carried out, and the result was satisfactory.
Upon cutting and sampling the steel pipe, it was discovered that the seepage is located in the weld area where cracks were found.

Fig. 1 Cracking Position and Morphology of Sulfuric Acid Pipe
To identify the cause of corrosion cracking and prevent the risk of recurrence, this article aims to analyze the chemical composition, metallographic microscope, and scanning electron microscope of the failed stainless steel pipe. By doing so, we can determine the root cause of the failure and suggest effective preventive measures.
The ARL-4460 direct reading spectrometer is utilized to detect the chemical composition of the base metal and weld of the stainless steel pipe, with the purpose of determining if they comply with the standard requirements.
Please cut a sample from the liquid penetration point as shown in Figure 1c. The sample should include the base metal, weld, and heat-affected zone. Next, perform pre-grinding, rough grinding, fine grinding, and polishing on the sample.
Afterward, utilize an OLYMPUS-GX51 metallographic microscope to observe any non-metallic inclusions present in the sample. Then, etch the sample with a ferric chloride hydrochloric acid aqueous solution. Finally, observe the sample’s structure under the metallographic microscope as shown in Figure 2.

Fig. 2 metallographic sample
Using hydraulic pliers, tear the sample along the crack, then scan and observe the crack surface using the Hitachi S-3400 thermal field emission scanning electron microscope. Next, conduct energy spectrum analysis with an EDAX energy spectrometer.
Table 1 displays the chemical composition of the base metal and weld of a stainless steel pipe.
As shown in Table 1, the chemical composition of the stainless steel pipe purchased by the company is lower than the standard for both base metal and weld metal. However, the content of other elements meets the standard requirements.
Chromium (Cr) is the primary corrosion-resistant element in stainless steel. When the Cr content is low, the corrosion resistance of stainless steel will decrease.
Table 1 Chemical Composition of Stainless Steel Pipe Materials (Mass Fraction (%)
| Element | C | Si | Mn | P | S | Cr | Ni |
| GB/T4237—2015 | ≤0.07 | ≤0.75 | ≤2.00 | ≤0.045 | ≤0.030 | 17.50~19.50 | 8.0~10.5 |
| Base metal | 0.07 | 0.39 | 0.99 | 0.033 | 0.011 | 17.36 | 10.14 |
| Weld bead | 0.07 | 0.40 | 1.00 | 0.030 | 0.013 | 16.85 | 10.03 |
Initially, the specimen underwent mechanical polishing, and non-metallic inclusions distribution was observed under a microscope without etching.
Upon observation, it was found that there were few non-metallic inclusions, but single, large-sized inclusions were present, rated as Ds2 (refer to Fig. 3a).
The presence of non-metallic inclusions can disrupt the matrix’s continuity, decrease its mechanical properties, and increase its susceptibility to fractures.
Non-metallic inclusions can also reduce the thickness of the passive film (oxide film) formed on the surface of the stainless steel substrate, leading to the corrosion of the junction between the inclusions and the substrate first. Subsequently, the local corrosion at the interface can extend to the substrate, resulting in pitting corrosion.
Furthermore, the presence of non-metallic inclusions can promote grain boundary embrittlement and intergranular corrosion, thus reducing the material’s corrosion resistance.


Fig. 3 Microstructure of Specimen Fracture after Corrosion
The polished sample underwent chemical etching, and its structure was observed using a metallographic microscope.
Fig. 3b depicts the micrograph of the sample’s base metal. The structure is single-phase austenite (with twins), with no abnormalities found at the grain boundary. The average grain size of the metal is grade 7.
Fig. 3c displays the microstructure of the fusion zone (left weld, right heat-affected zone). This zone exhibits a normal structure, good fusion, and no cracks, pores, or other welding defects.
Metallographic microstructure is observed near the crack (welding heat-affected zone), as shown in Fig. 3d. The microcracks distributed along the grain boundary are clearly visible, with carbide of network grain Cr, forming a chromium-poor zone, as shown in Fig. 4.
A chromium content (mass fraction) greater than 12% produces an obvious passivation effect, significantly improving the corrosion resistance of stainless steel. A chromium content less than 12% destroys the passivation state, causing a drop in potential, and the passivation state remains in the crystal, forming a micro galvanic cell with a small anode (chromium-poor area in the grain boundary area) and a large cathode (matrix). This accelerates the corrosion of the grain boundary.
The precipitation temperature of Cr23C6 carbide is 450-850 ℃, which is the sensitization temperature range of stainless steel intergranular corrosion, also known as the dangerous temperature range.
The above morphological features show that there is sensitization in this area after welding, leading to intergranular corrosion in the heat-affected zone of the weld and reducing the intergranular corrosion resistance of the stainless steel heat-affected zone. This is one of the reasons for the cracking of the stainless steel pipe.
Place the processed fracture sample in the scanning electron microscope to conduct microscopic observation and analysis using secondary electron imaging.
As depicted in Figure 4, it is evident that the fracture is uneven with numerous corrosion products and cracks distributed in a dendritic pattern.
The cracks exhibit secondary features and have penetrated the material matrix, indicative of stress corrosion cracking as the cause of failure in the 304 stainless steel pipe.
Stainless steel has low thermal conductivity, and welding generates residual stress due to high temperatures.
The corrosion microcracks in the stainless steel pipe accelerate under residual stress, leading to stress corrosion cracking.

Fig. 4 SEM observation of fracture morphology
The energy spectrometer was used to analyze the corrosion products on the fracture surface of the stainless steel pipe. Fig. 5 displays the results of the energy spectrum analysis.
From the diffraction peak spectrum, it is evident that the chlorine content is exceptionally high, indicating that the stainless steel pipe is exposed to a chlorine-containing corrosion environment.
The cracked steel pipes of the fertilizer plant are stored in an open-air location.
The plant site is situated in the coastal zone, just 1.1 km from the coast, which is a typical marine atmospheric environment.
During periods of high temperature and humidity, seawater evaporates in large quantities, producing salt fog that results in a high concentration of chloride ions in the air.
Water containing chloride ions is adsorbed onto the outer wall of the stainless steel pipe, forming a corrosive medium that continuously corrodes the stainless steel pipe.
Austenitic stainless steel naturally forms a dense passivation film (oxide film) on its surface in an ordinary atmospheric environment.
This passivation film insulates the atmosphere from direct contact with the stainless steel surface, providing excellent corrosion resistance and protection.
Even if the passive film is damaged, it can be regenerated and repaired in a timely manner.
However, chloride ions easily destroy the passivation film of austenitic stainless steel, leading to the formation of pitting or pits on the surface and accelerating the corrosion of the stainless steel.

The corrosion cracking of stainless steel pipes in this case cannot be attributed to a single factor. Instead, it is caused by the joint action of multiple factors.
(1) Non-metallic inclusions can damage the integrity of the passive film on the metal surface, reducing the corrosion resistance of stainless steel. Therefore, it is important to strictly control non-metallic inclusions below Level 1.5.
(2) The low Cr content in the base metal and weld metal reduces the compactness of the chromium passive film on the stainless steel surface. To improve the quality of steel pipes and welding materials, incoming components should be strictly tested to ensure that the weld metal composition is not weaker than the base metal.
During the welding process, welding parameters should be strictly controlled, and the welding heat input should be as small as possible to prevent sensitization, which can cause Cr to precipitate along the grain boundary and generate Cr23C6, leading to intergranular corrosion of stainless steel.
(3) The chemical fertilizer plant is located in a marine atmospheric environment, where the high chloride ion content in the air, appropriate temperature, and humidity accelerate corrosion. This causes the oxide film on the stainless steel surface to be easily damaged, resulting in electrochemical corrosion.
Corrosion microcracks expand rapidly under the effect of residual stress, leading to stress corrosion cracking.
Therefore, it is necessary to strictly control the site air environment and isolate the salt fog environment (for example, by painting or adding a protective layer) to prevent damage from chloride ions.