Why do oxygen and acetylene cylinders explode? The answer lies in a mix of physics and safety practices. These cylinders are essential in various industrial settings, but their potential for danger is high. This article explains the critical reasons behind these explosions, including improper handling, temperature changes, and exposure to certain elements. Readers will learn practical steps to safely manage these cylinders, minimizing the risk of catastrophic accidents. Dive in to discover vital safety measures that could protect you and your workplace.


Before installing the pressure reducing valve, slowly open the cylinder valve, blow off any dust inside and outside the interface, install the pressure reducing valve, and then open the valve for inspection.
◆ People should not face the interface at the side of the interface during operation.
◆ Check that the leather pipe joint is free of dust and metal chips before connecting.
◆ After removing the leather tube, do not place it up to the sky or on the ground to prevent impurities from entering.
◆ Do not completely deplete the oxygen in the bottle; reserve 1-1.5 atmospheres to facilitate air flushing inspection and prevent impurities from entering.
◆ In winter, only use hot water or steam to defrost the bottle valve. It is prohibited to use a flame for heating or an iron hammer.
◆ When working in the same area as an electric welder, insulating pads should be added at the bottom of the cylinder to prevent electrification of the cylinder.
◆ Metal pipes and equipment in contact with gas cylinders must be installed with grounding wires to prevent static electricity from causing fire and explosion.

There are four reasons:
There are several reasons why acetylene cylinders must be placed vertically.
Firstly, the cylinder contains acetone, which is a filler and solvent. When the cylinder is used horizontally, acetone can easily flow out with the acetylene gas, leading to increased acetone consumption and reduced combustion temperature.
This can cause backfire and lead to an explosion accident. Acetylene in the cylinder is dissolved in acetone solvent under pressure. When the valve is opened, the pressure decreases, and the dissolved acetylene becomes a gas and is released.
Acetylene cylinders placed horizontally may cause acetone to flow out, and this can quickly volatilize and mix with air to form an explosive mixture. The explosion limit is between 2.3% and 72.3% (vol), and the minimum ignition energy is 0.019mJ. In case of open fire and heat energy, combustion and explosion can occur.
Secondly, when acetylene cylinders are laid horizontally, they are prone to rolling and can easily impact other objects, creating excitation energy that can cause accidents.
Thirdly, acetylene cylinders are equipped with shockproof rubber rings to prevent collisions during loading, unloading, transportation, and use. The rubber ring is an insulating material, meaning that the acetylene cylinder must be placed on an electrical insulator horizontally to prevent the static electricity generated on the cylinder from spreading to the ground.
If static electricity gathers on the cylinder, it can easily generate static sparks. When acetylene gas leaks, it can lead to combustion and explosion accidents.
Finally, when using an acetylene cylinder, the valve must be equipped with a pressure reducer, flame arrester, and rubber tube. Since the cylinder is prone to rolling when lying down, it can easily damage the pressure reducer, flame arrester, or pull off the rubber tube, causing acetylene gas to leak out and leading to combustion and explosion accidents.
In conclusion, acetylene cylinders must be placed vertically to prevent accidents caused by acetone flow, rolling, static electricity, and equipment damage.
A: It’s not necessary.
Answer:Dumping the cylinder can cause the valve to dislodge, releasing the gas stored inside. The powerful force of the escaping gas can cause the cylinder to move rapidly forward or rotate on the ground, potentially injuring anyone in close proximity.
Moreover, if the gas is combustible, it can lead to an explosion, which could have even more severe consequences.
Acetylene is highly flammable, while oxygen is a combustion supporter.
If acetylene leaks, it can mix with air and cause a violent explosion upon contact with sparks or open flames. Such an explosion can cause damage to the oxygen cylinder and result in oxygen leakage.
The combustion-supporting property of oxygen can exacerbate the intensity of the explosion beyond control. Therefore, it is crucial to keep acetylene and oxygen separate and avoid placing them together.

The temperature of the acetylene cylinder should not exceed 40 ℃, and the boiling point of acetone is 58 ℃. As the temperature increases, the rate of acetone volatilization also increases. This can cause acetylene to separate, leading to a sharp increase in cylinder pressure.
To ensure safe usage, a small amount of pressure is left in the acetylene cylinder, which makes the pressure inside the cylinder greater than the pressure outside. This helps prevent the inflow of other gases. Since the explosion limit of acetylene is low, it can explode if mixed with even a little air and exposed to a certain temperature.
Therefore, it is crucial to have a pressure-reducing valve installed at the exhaust port of the cylinder to prevent air from mixing with acetylene. Otherwise, there could be a risk of explosion during the next use.
Using a pressure-reducing valve is also essential to maintain the air pressure inside the cylinder greater than that of the outside air, and to avoid any air from flowing back into the acetylene cylinder. In the case of an oxygen cylinder, it must retain a residual pressure of at least 0.098~0.196MPa gauge pressure.
For acetylene cylinders, a residual pressure of gauge pressure of 49Kpa~98KPa in winter and 196KPa in summer must be retained to ensure safety.
Oil, especially unsaturated and sour fat, is prone to vaporize and release heat, which is why oil gauze heads and oil cloths can self-ignite due to oxidation in the air, with heat unable to dissipate. Once it reaches the self-ignition point, self-ignition can occur.
However, oil vaporizes slowly in the air, and the heat generated dissipates quickly, making it generally difficult to accumulate heat and ignite spontaneously.
When oil and fat come into contact with pure oxygen, its gasification speed accelerates significantly, releasing a lot of heat that causes rapid temperature rise and combustion. Pure oxygen has strong oxidation properties that promote the violent combustion of combustibles.
If the oxygen cylinder mouth is contaminated with grease, the grease will be oxidized rapidly when the oxygen is sprayed out, and the heat generated by the friction between the high-pressure airflow and the mouth of the cylinder will further accelerate the oxidation reaction. This can cause the grease on the oxygen cylinder or the pressure-reducing valve to ignite and even explode.
Therefore, it is strictly prohibited for the oxygen cylinder, especially the mouth of the cylinder and the accessories in contact with oxygen, to come into contact with grease.

Most cylinder valves of steel cylinders are made of copper alloy, which is relatively fragile. Although some are made of steel, they have a smaller structure than the cylinder body and are rotated on the cylinder body to form a right angle between the bottleneck and the cylinder valve joint. These areas are both vulnerable and prominent points of the cylinder body and are most susceptible to mechanical damage or external impact during handling, storage, and use.
If the cylinder falls, rolls, or is hit by other hard objects due to careless damage, the joint of the cylinder valve and bottleneck is easily broken, leading to severe consequences. If the oxygen bottle valve is broken, the high-pressure gas (150 kg/cm2) in the bottle will be ejected, causing the cylinder to rush in the opposite direction and potentially damaging machinery, equipment, and buildings, or even causing casualties.
If the acetylene cylinder valve is broken, the flammable gas rushes out, forming an explosive gas mixture with the air, which may explode when encountering an open fire. Additionally, if the cylinder contains combustible gas, the static electricity generated by the high-speed jet or other ignition sources may cause combustion and explosion.
Furthermore, when the bottle valve is exposed, it is susceptible to the invasion of dust or grease substances during handling and storage, creating a potential danger. Wearing a safety helmet can prevent contamination and intrusion of dust or grease.
To eliminate these hazards, the cylinder making unit must equip the cylinder with a safety helmet before leaving the factory. When using gas, unscrew the safety helmet and place it in a fixed place. After use, put on and tighten the bottle cap promptly and avoid throwing it around. Never forget to wear the safety helmet during handling.
The collision can result in the breakdown of activated carbon, leading to an increase in the expansion space. This, in turn, causes acetylene gas to accumulate under high pressure, creating a risk of an explosion. Furthermore, with an increase in temperature, the gaseous acetylene can polymerize, resulting in an explosion.
Answer: Due to the severe collision or impact on the gas cylinder, an explosion accident will occur and the consequences will be very serious.
Oxygen cylinders are high-pressure containers that can easily explode if dropped carelessly.
When transporting multiple oxygen cylinders, a special container designed for one-time use is recommended. This container is suitable for loading and unloading the cylinders safely.

The explosion of an acetylene cylinder is primarily caused by a rapid increase in temperature and pressure and the decomposition of acetylene.
The following are characteristics of acetylene decomposition:
If the temperature of the bottle wall rises (starting from the top of the bottle) or if an abnormal odorous gas with smoke escapes from the open bottle valve after tempering occurs, it indicates that acetylene has started to decompose. If the acetylene cylinder is exposed directly to flame or radiant heat, there is a risk of acetylene decomposition at any moment.
Reasons for acetylene decomposition:
(1) Welding tempering;
(2) External heating (burning substances are near the acetylene cylinder, and tools such as welding guns or cutting guns that have not been extinguished are hung on the cylinder);
(3) Acetylene near the cylinder valve or pressure reducer is on fire;
(4) Severe shock or vibration.
Precautions:
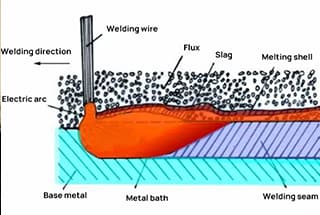
Oxygen pipes are designed for high-pressure applications, while acetylene pipes are intended for low-pressure use.
Furthermore, during the use of acetylene pipes, there may be occasional slight flashbacks, and carbon deposits may accumulate in the pipes. If these deposits mix with oxygen, they can potentially cause an explosion.
Answer: If the cylinder is filled with other gases, severe explosion will occur, with very serious consequences.
Answer: To avoid electrification of gas cylinders.
When working with an electric welder (assuming this is the context), it is important to pad the bottom of the oxygen cylinder with insulating materials to prevent it from becoming electrified.
Additionally, any metal equipment, such as pipes, that comes into contact with gas cylinders should have good grounding devices to prevent accidents caused by static electricity, such as burning or explosions.
The ignition energy required to ignite acetylene is a mere 0.019 mJ. This means that even a small electrostatic discharge, typically several mJ, can cause acetylene to ignite or even detonate.
When acetylene flows or leaks through a gas transmission pipe, it generates static electricity. Any form of electrostatic discharge can trigger an ignition.
Once ignited, acetylene combustion and explosion can occur without the need for oxygen, making explosion highly probable.
To prevent explosions, it is advisable to ground the acetylene cylinder directly. By doing so, the cylinder will not accumulate static electricity, thereby significantly reducing the risk of explosion.
Prolonged exposure of acetylene to copper and silver can lead to the formation of explosive compounds, namely copper acetylide and silver acetylide. These compounds can trigger an explosion under extreme vibration or when exposed to temperatures between 110-120 ℃.