What if the key to unlocking the secrets of steel lies in understanding its tiniest structures? In this article, we’ll explore how to identify ferrite and retained austenite in steel, crucial for enhancing material performance. Learn to distinguish these structures and improve your metallurgical skills!
I’d like to share with you the identification of “Ferrite” and “Retained Austenite”, as part of a series of articles on the identification of similar structures in steels.
Since Ferrite and Retained Austenite are not corroded, they both appear white when observed under a microscope. However, they can be easily confused if not properly observed.
Fortunately, it’s relatively easy to distinguish between the two structures by mastering certain methods. Two common methods include:
Ferrite and Retained Austenite often co-exist in the microstructure of hypoeutectoid steel that has undergone quenching. There are typically three forms of ferrite in these quenched parts: polygonal undissolved ferrite, massive proeutectoid ferrite, and reticular or semi-reticular proeutectoid ferrite. All of these forms of ferrite are white and bright in appearance.
The polygonal and massive ferrites have well-defined boundaries and are often found in the blank areas between the needles of martensite. Upon closer inspection, the white phase and the martensite phase can be seen to be on the same plane.
The reticular or semi-reticular ferrite is finely distributed along the original austenite grain boundary.
Retained Austenite, on the other hand, lacks well-defined boundaries, and its shape changes with the shape of the martensite needle distribution. It usually does not exist alone, but is organically combined with the needle-like martensite after quenching. As a result, its color is slightly darker than that of ferrite, and the phenomenon of needle-like martensite is often faintly visible.
If the quenching heat preservation time for hypoeutectoid steel is insufficient or the temperature is too low, white polygonal undissolved ferrite will appear in the resulting microstructure.

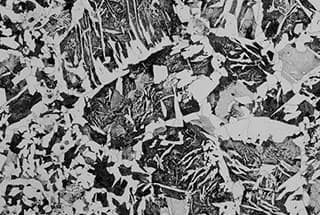
Fig. 1 white polygonal undissolved ferrite
As illustrated in Fig. 1, the microstructure of 45 steel that has undergone water quenching at 760 ℃ for 25 minutes consists of white polygonal undissolved ferrite, black medium carbon quenched martensite, light gray martensite, and a residual austenite matrix.
If there are many workpieces in the furnace and the tapping time is excessive, the cooling rate of the workpieces will be greater than the cooling rate in an annealing furnace but less than the normalizing air cooling rate. Alternatively, if the workpieces are left in the air for too long after tapping, the resulting microstructure will contain massive proeutectoid ferrite.
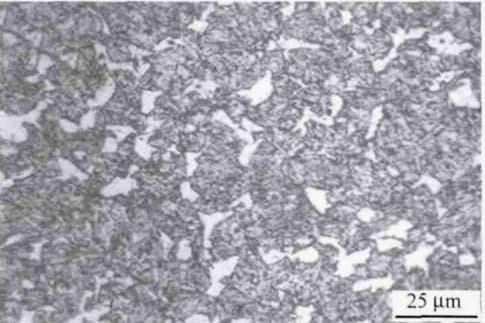
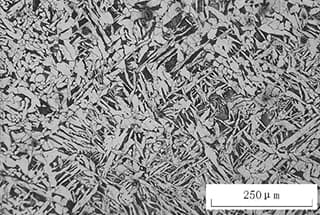
Fig. 2 white massive proeutectoid ferrite
As depicted in Fig. 2, the microstructure of 45 steel was obtained after heating at 840°C for 25 minutes, followed by water quenching and then tempering at 600°C for 60 minutes. The white massive structure is eutectoid ferrite, while the remaining structure is tempered sorbite.
This result was due to multiple workpieces present in the heating furnace during the test, and the furnace door was not kept closed during quenching, as required. Instead, the furnace door was kept open after the first sample was quenched and until the last sample was quenched.
As a result, in the later stages of quenching, approximately half of the quenched samples showed massive proeutectoid ferrite. This amount increased from less to more with the prolongation of the quenching time, with the content of massive proeutectoid ferrite in the last quenched sample reaching as high as 40% (volume fraction).
Due to the open furnace door, when the temperature of the workpieces in the furnace was below AC3, the cooling rate of the workpieces was higher than that of cooling (equivalent to annealing) but lower than that of air cooling (equivalent to normalizing). This resulted in the precipitation of massive proeutectoid ferrite.
If the quenching cooling rate was not sufficient, the proeutectoid ferrite in the steel was generally distributed along the original austenite grain boundary in the form of a network or semi-network.

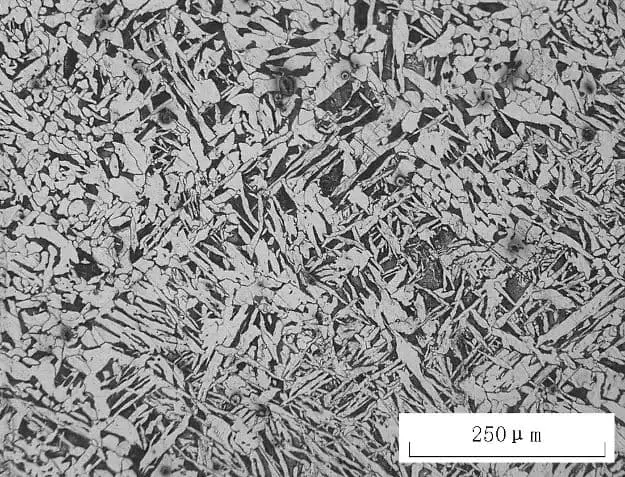
Fig. 3 white reticular proeutectoid ferrite
As shown in Fig. 3, the microstructure of 45 steel after heating at 900°C for 25 minutes and oil quenching consists of white fine mesh pre-eutectoid ferrite, black quenched troostite, feathery upper bainite, light gray martensite, and a residual austenite matrix.
The residual austenite, which is not on the same plane as the martensite, is only visible in the quenched structure when the quenching heat is severely overheated. In normal quenching, the residual austenite is not prominently present.

Fig. 4 white retained austenite
As shown in Fig. 4, the microstructure of 45 steel after heating at 900°C for 25 minutes and water quenching consists of black quenched medium carbon martensite and white residual austenite.
The shape of the residual austenite changes depending on the angle at which it intersects with the martensite.
In this post, we present the methods for identifying ferrite and retained austenite. We hope that you will find this information useful.
It should also be noted that a thorough understanding of the iron-carbon phase diagram, combined with the perspectives discussed in the article, will make the identification process much easier.


