Why does steel change its structure under different conditions? This article explores the five critical factors that influence the martensite start (Ms) point in steel. From chemical composition and deformation stress to quenching rates, austenitizing conditions, and even magnetic fields, these variables dictate the transformation behavior of steel. By understanding these factors, you’ll gain insight into optimizing steel’s mechanical properties for various applications. Read on to discover how each element impacts steel’s performance.
Generally speaking, Ms point mainly depends on the chemical composition of the steel, of which the carbon content has the most significant effect.
With the increase of carbon content in the steel, the temperature range of martensitic transformation decreases, as shown in Fig. 1.
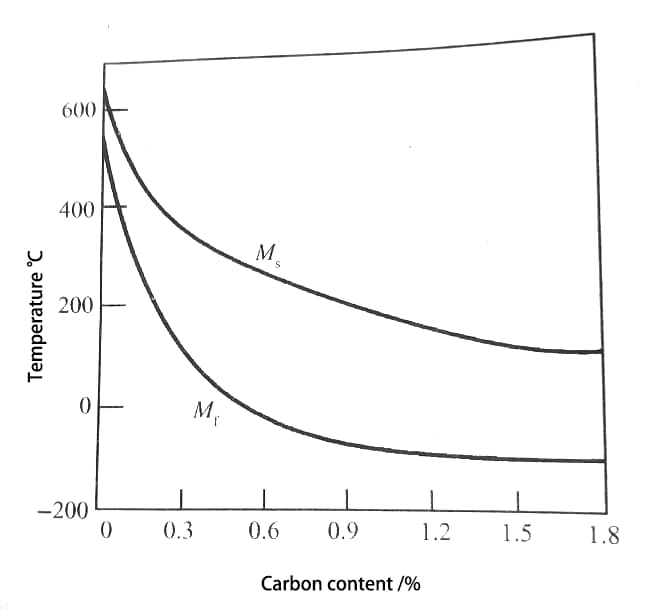
Fig. 1 Effect of Carbon Content on Ms and Mf
With the increase of carbon content, the changes of Ms point and Mf point are not completely consistent, and Ms point shows a relatively uniform continuous decline;
When the carbon content is less than 0.6%, the Mf point decreases more significantly than the Ms point, thus expanding the temperature range of martensitic transformation (Ms Mf).
However, when the carbon content is greater than 0.6%, the Mf point decreases slowly, and because the Mf point has dropped below 0 ℃, there is more residual austenite in the room temperature structure after quenching.
The effect of N on Ms point is similar to that of C.
Like C, N forms interstitial solid solution in steel, which has solid solution strengthening effect on phase γ and phase α, but especially on phase α, thus increasing the shear resistance of martensitic transformation and increasing the driving force of transformation.
At the same time, C and N are also elements that stabilize a phase.
They reduce the equilibrium temperature T0 of γ → α ‘phase transition, so they strongly reduce the Ms point.
The common alloying elements in steel can reduce Ms point, but the effect is not as significant as that of carbon.
Only Al and Co raise Ms point (as shown in Fig. 2).
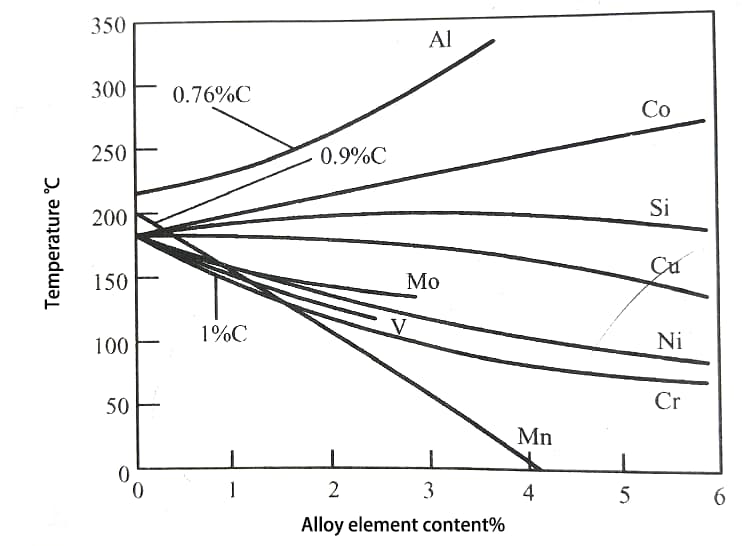
Fig. 2 Effect of alloy elements on Ms point of ferroalloy
The elements reducing Ms point are arranged in the order of their influence intensity: Mn, Cr, Ni, Mo, Cu, W, V, Ti.
Among them, W, V, TI and other strong carbide forming elements mostly exist in the form of carbides in steel, and they are rarely dissolved in austenite during quenching and heating, so they have little effect on Ms point.
The influence of alloying elements on Ms point mainly depends on their influence on the equilibrium temperature T0 and the strengthening effect on austenite.
All elements (such as C) that sharply reduce T0 temperature and strengthen austenite sharply reduce Ms point.
Mn, Cr, Ni, etc. not only reduce the T0 temperature but also slightly increase the austenitic strength, so they also reduce the Ms point.
Al, Co, Si, Mo, W, V, Ti, etc. all increase the T0 temperature, but also increase the austenite strength to varying degrees.
So,
① If the former plays a greater role, the Ms point will rise, such as Al and Co;
② If the latter has a greater effect, the Ms point will be lowered, such as Mo, W, V, Ti;
③ When the two functions are roughly equivalent, it has little effect on Ms point, such as Si.
In fact, the interaction between alloy elements in steel is very complex, and the Ms point of steel mainly depends on test.
It is generally believed that all alloying elements that reduce Ms point also reduce Mf point.
As previously mentioned, martensitic transformation will be induced when the austenite is plastically deformed between Md Ms.
Similarly, plastic deformation between Ms Mf can also promote martensitic transformation and increase martensitic transformation.
In general, the larger the deformation and the lower the deformation temperature, the more deformation induced martensite transformation variables.
Since martensite transformation will inevitably produce volume expansion, multi-directional compressive stress will prevent the formation of martensite, thus reducing Ms point.
However, tensile stress or unidirectional compressive stress is often conducive to the formation of martensite, which makes Ms point rise.
The influence of heating temperature and holding time on Ms point is complex.
The increase of heating temperature and the extension of holding time are conducive to the further dissolution of carbon and alloy elements into austenite, which will reduce the Ms point, but at the same time, it will cause the growth of austenite grains, reduce its crystal defects, and reduce the shear resistance during the formation of martensite, thus increasing the Ms point.
In general, if there is no change in chemical composition, that is, under the condition of complete austenitizing, increasing the heating temperature and prolonging the holding time will increase the Ms point;
Under the condition of incomplete heating, increasing the temperature or prolonging the time will increase the content of carbon and alloy elements in austenite, leading to the decrease of Ms point.
Under the condition that the austenite composition is constant, the austenite strength will be increased and the shear resistance of martensitic transformation will be increased when the grain is refined, which will reduce the Ms point.
However, when the grain refinement does not significantly affect the shear resistance, it has little effect on Ms point.
The influence of the quenching cooling rate on Ms point is shown in Fig. 3.

Fig. 3 Effect of Quenching Speed on Ms Point of Fe-0.5% C-2.05% NI Steel
When the quenching speed is low, Ms point remains constant, forming a lower step, which is equivalent to the nominal Ms point of steel.
When the quenching speed is very high, another step occurs where Ms point remains constant.
Between the above two quenching speeds, Ms point increases with the increase of quenching speed.
The above phenomena can be explained as follows:
It is assumed that the distribution of C in austenite during phase transformation is uneven, and segregation occurs at defects such as dislocations, forming “C atomic air mass”.
The size of this “air mass” is related to temperature.
Under high temperature, the atomic diffusion ability is strong, and the tendency of C atom segregation is small, so the size of the “air mass” is also small.
However, when the temperature decreases, the atomic diffusivity decreases, the tendency of C atoms to segregate increases, and the inner “air mass” size increases with the temperature decreasing.
Under normal quenching conditions, these “air masses” can reach enough size to strengthen austenite.
However, the extremely fast quenching speed inhibits the formation of “air mass”, which leads to the weakening of austenite and the reduction of shear resistance during martensitic transformation, thus raising Ms point.
However, when the cooling rate is high enough, the “air mass” bending is restrained, and Ms point no longer increases with the increase of quenching rate.
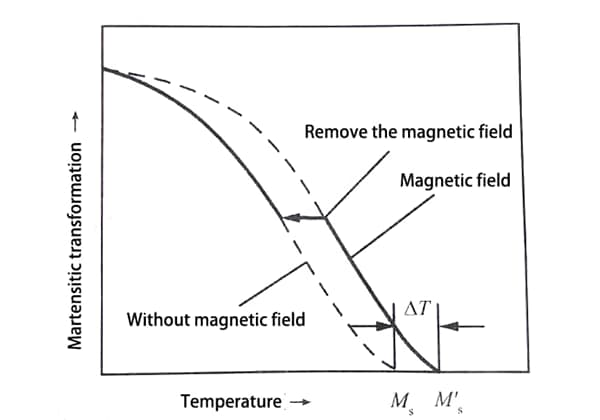
The test shows that when the steel is quenched and cooled in the magnetic field, the applied magnetic field will induce the martensite transformation.
Compared with that without the magnetic field, the Ms point increases, and the martensite transformation at the same temperature increases.
However, the external magnetic field only makes Ms point rise, but has no effect on the phase transition behavior below Ms point.

Fig. 4 Effect of external magnetic field on the martensite transformation process
As shown in Fig. 4, the applied magnetic field increases Ms to Ms’ during quenching and cooling, but the increasing trend of the rotational variable is basically consistent with that without magnetic field.
When the applied magnetic field is removed before the phase transformation is over, the phase transformation will immediately return to the state when the magnetic field is not applied, and the final transformation amount of martensite will not change.
The reason why the external magnetic field affects the martensite transformation is that the external magnetic field makes the martensite phase with the maximum magnetic saturation strength more stable.

Fig. 5 Thermodynamic diagram of Ms point rise caused by external magnetic field
As shown in Fig. 5, the free energy of martensite decreases in the magnetic field, while the magnetic field has little effect on the free energy of non ferromagnetic austenite.
Therefore, the two-phase equilibrium temperature T0 increases, and the Ms point also increases. It can also be considered that the external magnetic field actually compensates part of the chemical driving force with magnetic energy, and the martensitic transformation can occur above Ms point due to magnetic induction.
This phenomenon is very similar to deformation induced martensitic transformation from the thermodynamic point of view.
Through the introduction of this issue, we should be clear about the five factors that affect Ms points.
Of course, regular review of these knowledge points will also play a beneficial role in our understanding of knowledge points.