What sets galvanneal and galvanized steel apart? While both are treated to prevent rust, their unique coating processes result in different properties. This article dives into their distinctions, comparing aspects like corrosion resistance, surface finish, and applications. By the end, you’ll understand which material suits your needs best, whether for durability or specific industrial uses.
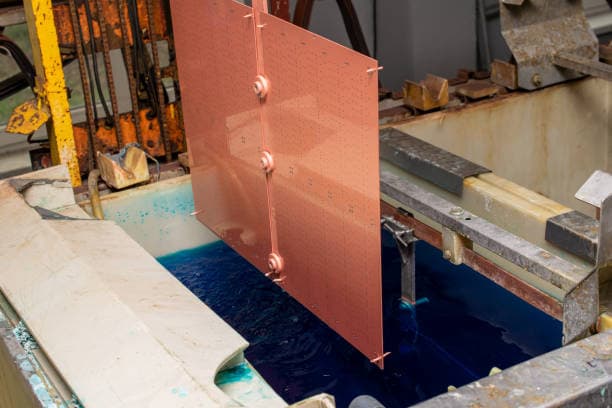
Electro-galvanizing, commonly referred to as cold galvanizing, is an electrochemical process in which zinc ingots serve as anodes and steel strips as cathodes.
The process involves the zinc atoms losing electrons and becoming ions, which dissolve in the electrolyte. The steel strips, serving as cathodes, then receive electrons from the zinc ions, reducing them back into zinc atoms and depositing them on the surface of the steel strips to form a plating layer.

There is a significant difference in the amount of galvanizing between hot-dip galvanized sheets and electro-galvanized sheets.
The amount of galvanizing in hot-dip galvanized sheets cannot be too low.
Typically, the minimum is 50-60g/m2 on both sides, with a maximum of 600g/m2.
The electro-galvanized sheet has a very thin galvanized layer, with a minimum of only 15g/m2.
However, if a thicker coating is required, the production speed is slow, making it unsuitable for modern production processes.
Typically, the maximum amount of galvanizing for electro-galvanized sheets is around 100g/m2.
Due to these limitations, the production of electro-galvanized sheets is significantly limited.

Expert 1 opinion
There are fundamental differences in the coating structure between hot-dip galvanized sheets and electro-galvanized sheets.
The hot-dip galvanized sheets have a slightly brittle compound layer between the pure zinc coating and the steel strip substrate. Most of the pure zinc coating forms zinc flowers during crystallization, resulting in a uniform coating without pores.
In contrast, the zinc atoms of the electro-galvanized layer are only deposited on the surface of the steel strip through physical action. This leaves many air drying holes, making it susceptible to pitting corrosion from corrosive media.
As a result, hot-dip galvanized sheets are more corrosion resistant than electro-galvanized sheets.
The production processes for hot-dip galvanized and electro-galvanized sheets are also quite different. Hot-dip galvanized sheets are typically made from cold-rolled sheets that are annealed and continuously hot-dip galvanized on the galvanizing line. The rapid heating and cooling of the steel strip affects its strength and plasticity to some extent and results in a lower stamping performance compared to cold-rolled sheets that are degreased and annealed on professional production lines.
On the other hand, electro-galvanized sheets are made from cold-rolled sheets and have the same processing performance as cold-rolled sheets. However, the complex production process increases the cost.
In conclusion, hot-dip galvanized sheets have a lower production cost and a wider range of applications, making it the main variety in the galvanized sheet market.
Expert 2 opinion
Electro-galvanizing is a process of galvanizing through electrode reaction, while hot-dip galvanizing involves dipping raw materials into a zinc pot for galvanizing, which involves both inter-metallic reactions and physical reactions.
The surface of raw materials in hot-dip galvanizing is comprised of a layer of inter-metallic compounds followed by zinc, whereas the surface of electro-galvanizing is just zinc without an intermediate layer.
The zinc layer in electro-galvanizing is thin, whereas the zinc layer in hot-dip galvanizing is thick.
Electro-galvanizing has limited production capacity and a low output, while hot-dip galvanizing has a high production capacity and output.
The surface finish and corrosion resistance of electro-galvanizing is better than that of hot-dip zinc, making it more expensive. The cost of electro-galvanizing is high, leading to a higher price. When the price of zinc-aluminum alloy for hot-dip galvanizing is high, it can exceed 4,000 yuan per ton.
Despite having the same anti-corrosion principle, the processes of electro-galvanizing and hot-dip galvanizing are different. After galvanizing, the surface is smooth and bright, but over time, the zinc layer may fall off.
Hot-dip galvanizing may not be as visually appealing as electro-plating, but the zinc layer has deeper penetration, making it more durable over time.
Expert 3 opinion
(1) Typically, the zinc layer of hot-dip galvanized sheet is thicker, around 10um or more, and has excellent corrosion resistance, making it a widely used zinc plating process.
In comparison, the zinc layer in electro-galvanizing is very thin, around 3-5um. The surface of hot-dip galvanizing is rough and bright, and in severe cases, it may have zinc flowers. On the other hand, the surface of electro-plating is smooth and dark (dirty).
Electro-plated galvanized steel has good processability, but its thin coating and lower corrosion resistance make it less desirable compared to hot-dip galvanized steel sheet.
Cold galvanized sheets are electro-galvanized with a small amount of zinc, only galvanized on the outer wall of pipes (hot-dip galvanizing covers both the inside and outside). The amount of zinc is only 10-50g/m2, resulting in much lower corrosion resistance compared to hot-dip galvanized pipes.
(2) Chemical industry often uses electroless galvanizing, which is suitable for small parts.
In contrast, hot-dip galvanized sheets are usually used for power equipment and components and are suitable for large parts and equipment.
Electro-galvanizing, commonly known as cold galvanized sheet, is an electrochemical method in which zinc ingots are used as anodes, causing zinc atoms to lose electrons and become ions that dissolve into the electrolyte. The steel strip acts as the cathode and the zinc ions receive electrons and are reduced into zinc atoms, depositing on the surface of the steel strip and forming a coating.
The process of hot-dip galvanizing starts with pickling the steel pipe to remove the iron oxide on its surface. After pickling, the steel pipe is cleaned in a tank containing an aqueous solution of ammonium chloride, zinc chloride, or a mixture of the two. Then, the steel pipe is sent to the hot-dip galvanizing tank.
(3) There is a significant difference in the amount of galvanizing between hot-dip galvanized sheets and electro-galvanized sheets.
The amount of galvanizing for hot-dip galvanized sheets cannot be too small and typically ranges from a minimum of 50-60g/m2 on both sides to a maximum of 600g/m2.
On the other hand, the galvanized layer of electro-galvanized sheets can be very thin, with a minimum of 15g/m2. However, if the coating needs to be thick, the production line speed becomes very slow, making it unsuitable for the fast-paced processes of modern units. Generally, the maximum amount of galvanizing for electro-galvanized sheets is about 100g/m2.
This limitation in the production of electro-galvanized sheets greatly restricts its use.
(4) There are fundamental differences in the coating structure between hot-dip galvanized sheets and electro-galvanized sheets.
Hot-dip galvanized sheets have a slightly brittle compound layer between the pure zinc coating and the steel strip substrate. Most of the pure zinc coating forms zinc flowers during crystallization, creating a uniform coating without pores.
On the other hand, the zinc atoms of the electro-galvanized layer are only deposited on the surface of the steel strip through physical action. This leads to many air drying holes, making it more susceptible to pitting corrosion from corrosive media.
As a result, hot-dip galvanized sheets have better corrosion resistance compared to electro-galvanized sheets.
(5) The heat treatment processes for hot-dip galvanized sheets and electro-galvanized sheets are also significantly different.
Hot-dip galvanized sheets are typically made from cold-rolled sheets and are continuously annealed and hot-dip galvanized on the galvanizing line. The rapid heating and cooling of the steel strip affects its strength and plasticity to some extent, leading to a lower stamping performance compared to cold-rolled sheets that have been degreased and annealed on a professional production line.
On the other hand, electro-galvanized sheets are made from cold-rolled sheets and have similar processing performance to cold-rolled sheets. However, the complex production process increases the cost.
In conclusion, hot-dip galvanized sheets have a lower production cost and a wider range of applications, making it the dominant variety in the galvanized sheet market.
(6) Hot-dip galvanized sheet pipes are a type of alloy layer formed by the reaction between molten metal and iron matrix, which combines the matrix and coating.
Hot-dip galvanized sheets have the advantages of uniform coating, strong adhesion, and long service life. To ensure quality, most regular galvanized pipe manufacturers do not use electro-galvanizing (cold plating). Only small enterprises with limited scale and outdated equipment use electro-galvanizing, and as a result, their prices are relatively cheap.
The Ministry of Construction has officially declared that the use of outdated technology in cold galvanized sheet pipes will be phased out and it is prohibited to use cold galvanized sheet pipes as pipes for water and gas.
Nowadays, hot-dip galvanized sheets are widely used, while cold galvanized sheets are still used for electrical wire trunking, with slight color differences.

Hot Dip Galvanizing
Hot dip galvanizing involves immersing the workpiece in a molten zinc solution after it has undergone degreasing, pickling, and drying. The workpiece is left in the solution for a specific duration before being removed.
Cold Galvanizing
Cold galvanizing, also referred to as electro galvanizing, involves using electrolytic equipment to degrease and pickle the workpiece and then place it in a solution containing zinc salt. The negative electrode of the electrolytic equipment is connected to the workpiece, and a zinc plate is placed on the opposite side and connected to the positive electrode of the equipment.
When the power is turned on, the current flows from the positive electrode to the negative electrode, causing a layer of zinc to be deposited onto the workpiece.
Zinc Electroplating
Electro galvanizing has a relatively smooth and bright appearance. The plating layer obtained using the color passivation process is mainly yellow-green in color and displays a range of colors.
The plating layer obtained through the white passivation process is either cyan-white or white-green in color, and has a slight iridescent effect when viewed at a certain angle in sunlight.
Complex workpieces may experience “burning” around the corners and edges, resulting in a thick zinc layer that appears gray. Dead angles of current at internal corners can also lead to under-current gray areas where the zinc layer is thin.
The final product is free of zinc lumps and caking.
Hot Dip Galvanizing
The appearance of hot-dip galvanizing is slightly rougher and has a silvery-white color compared to electro galvanizing. It may also exhibit process water marks and a few drops, particularly at one end of the workpiece.
However, the zinc layer in hot-dip galvanizing is several times thicker than in electro galvanizing and offers several times greater resistance to corrosion.
The steel pipe can rust when exposed to air and reacts with oxygen or when it is eroded by oxygen in water.
To prevent this, a layer of galvanization is applied to the steel pipe, forming a galvanized layer that isolates it from the air and makes it more resistant to corrosion and rust.
Will Galvanized Steel Pipes Rust?
It’s not uncommon for galvanized steel pipes to rust over time, even though they are less susceptible to rust than ungalvanized pipes. The length of time before rusting occurs depends on various factors, including the environment and the conditions in which the pipe is used. For example, if the environment is humid or the pipe is exposed to rain for an extended period, it may become oxidized and develop spots, eventually leading to rust.
There are two types of galvanized steel pipes: hot galvanized and cold galvanized. Hot galvanized pipes are generally less prone to rust than cold galvanized pipes.

The rate of corrosion for galvanized pipes is largely influenced by factors such as the method of galvanization, thickness of the galvanized layer, amount of galvanization, and the environment in which the pipes are used.
Cold galvanized pipes are particularly susceptible to corrosion. They have a low amount of galvanization, with only the exterior of the steel pipe being coated, leaving the interior exposed. With an average of only 20g/m2 of zinc on one side, these pipes are prone to rusting.
Under normal conditions, cold galvanized steel pipes are expected to start rusting within a year. The rusting time can be prolonged by increasing the thickness of the galvanized layer. For example, pipes with a thickness of less than 2.75mm may take 2-3 years to start rusting, while those with a thickness of 3.0mm or more can last for 3-5 years before rusting begins.
If a galvanized pipe has become rusty, it can be treated to remove the rust. The following are the main methods for removing rust from galvanized pipes:
After the steel pipe undergoes galvanization, its surface is coated with a layer of zinc, which acts as a barrier between the steel pipe and the atmosphere. This prevents direct contact between the atmosphere and the steel pipe and protects it from corrosion.
The zinc coating on the steel pipe surface is highly reactive, and at normal temperatures, a thin and dense layer of zinc carbonate is formed in the air to protect the zinc from oxidation.
As a result, the galvanized pipe is protected from rust and does not require painting with anti-rust paint, whether it is the zinc coating or the steel pipe itself.
However, if the zinc coating is damaged (for example, when the steel pipe is welded and the coating at the joint burns off), the steel pipe is exposed to the air and loses the protection of the zinc coating. In this case, it must be repainted with anti-rust paint.
The galvanized layer of the galvanized steel pipe has anti-rust properties and is typically connected using threads.
In general, it is not necessary to paint with anti-rust paint unless unconventional methods of connection, such as welding, are used. If the galvanized layer is damaged, the affected area should be painted with anti-rust paint.