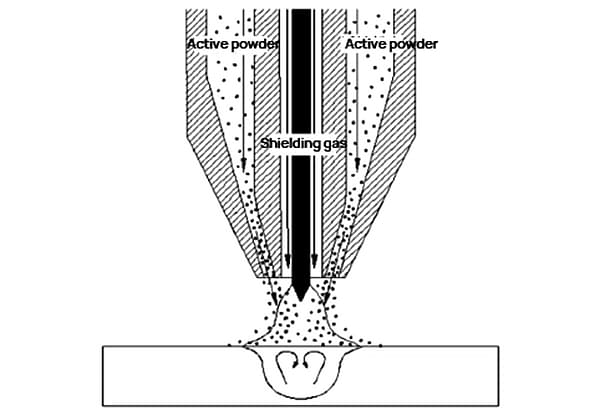
How do the various metal elements in welding wire impact the final weld quality? From silicon to vanadium, each element plays a unique role in the welding process. Understanding their effects can significantly improve weld strength, ductility, and resistance to defects. This article explores the functions and implications of these elements, providing essential insights for achieving optimal welding results.

Regarding the alloy elements such as Si, Mn, S, P, Cr, AI, Ti, Mo, and V contained in welding wire, what is the impact of these alloy elements on welding performance? The following are explanations for each element:

Silicon is the most commonly used deoxidizing element in welding wire. It can prevent iron from combining with oxygen and can reduce FeO in the molten pool.
However, when silicon is used alone for deoxidation, the resulting SiO2 has a high melting point (approximately 1710℃), and the particles produced are small and difficult to float out of the molten pool. This can lead to the entrapment of slag in the weld metal.
The function of manganese is similar to that of silicon, but its deoxidation ability is slightly inferior to silicon. When manganese is used alone for deoxidation, the resulting MnO has a higher density (15.11g/cm3) and is also difficult to float out of the molten pool.
In addition to its deoxidation function, manganese in welding wire can also combine with sulfur to form manganese sulfide (MnS), which can be removed (desulfurized), thereby reducing the tendency of hot cracking caused by sulfur.
Since it is difficult to remove the deoxidation products when silicon or manganese is used alone for deoxidation, a combination of silicon and manganese is commonly used to form a composite of silicate (MnO·SiO2) during deoxidation.
MnO·SiO2 has a lower melting point (approximately 1270℃) and a lower density (approximately 3.6g/cm3). It can coagulate into large blocks of slag and float out of the molten pool, achieving a good deoxidation effect.
Manganese is also an important alloy element in steel and an important hardenability element. It has a significant impact on the toughness of the weld metal.
When the Mn content is less than 0.05%, the weld metal has high toughness. When the Mn content is greater than 3%, the weld metal becomes brittle. When the Mn content is between 0.6% and 1.8%, the weld metal has high strength and toughness.
Sulfur in steel is often present in the form of iron sulfide, which is distributed in a mesh pattern along the grain boundaries and significantly reduces the toughness of the steel. The eutectic temperature of iron and iron sulfide is relatively low (985℃).
Therefore, during hot working, since the working starting temperature is generally between 1150-1200℃, the eutectic of iron and iron sulfide has already melted, leading to cracking during the working process.
This phenomenon is known as “sulfur hot brittleness.” The property of sulfur makes steel prone to hot cracking during welding.
Therefore, the sulfur content in steel is strictly controlled. The main difference between ordinary carbon steel, high-quality carbon steel, and advanced high-quality steel is the amount of sulfur and phosphorus content.
As mentioned earlier, manganese has a desulfurizing effect because it can form high-melting-point (1600℃) manganese sulfide (MnS) with sulfur, which is distributed in a granular form within the grains.
During hot working, manganese sulfide has enough plasticity to eliminate the harmful effects of sulfur. Therefore, maintaining a certain amount of manganese content in steel is beneficial.
Phosphorus can be completely dissolved in ferrite in steel. Its strengthening effect on steel is second only to carbon, and it increases the strength and hardness of steel.
Phosphorus can also improve the corrosion resistance of steel, but significantly decreases its ductility and toughness. This effect is particularly severe at low temperatures, which is known as the “phosphorus cold brittleness” phenomenon.
Therefore, it is detrimental to welding and increases the susceptibility of steel to cracking. As an impurity, the content of phosphorus in steel must also be restricted.
Chromium can increase the strength and hardness of steel while decreasing its ductility and toughness to a lesser extent. Chromium has strong corrosion and acid resistance capabilities, so austenitic stainless steels generally contain more than 13% chromium.
Chromium also has strong anti-oxidation and heat resistance properties. Therefore, chromium is also widely used in heat-resistant steels, such as 12CrMo, 15CrMo, and 5CrMo. Chromium is present in steel in certain amounts.
Chromium is an important component of austenitic steel and a component of ferrite. It can improve the oxidation resistance and mechanical properties of the alloy steel at high temperatures. In austenitic stainless steel, when the total amount of chromium and nickel is 40%, and the Cr/Ni ratio is 1, there is a tendency for hot cracking.
However, when the Cr/Ni ratio is 2.7, there is no tendency for hot cracking.
Therefore, in general, when the Cr/Ni ratio is around 2.2-2.3 in 18-8 type steel, chromium can easily form carbides in alloy steel, reduce thermal conductivity, and cause difficulties in welding due to the formation of chromium oxide.
Aluminum is one of the strong deoxidizing elements. Therefore, using aluminum as a deoxidizer can not only reduce the production of FeO but also make it easier to reduce FeO, effectively suppressing the chemical reaction of CO gas produced in the molten pool and improving the ability to resist CO porosity.
Additionally, aluminum can also combine with nitrogen to form a nitrogen fixation effect, reducing nitrogen porosity.
However, using aluminum for deoxidation results in the formation of high-melting-point AI2O3 (approximately 2050℃), which exists in a solid-state in the molten pool and is easy to cause slag entrapment in the weld metal.
At the same time, aluminum-containing welding wire is prone to spatter, and excessive aluminum content can reduce the weld metal’s resistance to hot cracking.
Therefore, the aluminum content in welding wire must be strictly controlled and should not be too high. If the aluminum content in the welding wire is properly controlled, the hardness, yield point, and tensile strength of the weld metal are slightly improved.
Titanium is also a strong deoxidizing element and can also combine with nitrogen to form TiN, playing a nitrogen fixation role, and improving the weld metal’s ability to resist nitrogen porosity.
If the appropriate amounts of titanium and boron (B) are present in the weld metal’s structure, the weld metal’s structure can be refined.
Molybdenum can increase the strength and hardness of alloy steel, refine grain size, prevent temper brittleness and overheating tendency, and improve high-temperature strength, creep strength, and durability.
When the content of molybdenum is less than 0.6%, it can improve ductility, reduce the tendency to crack, and increase impact toughness. Molybdenum also has a tendency to promote graphitization.
Therefore, the molybdenum content in heat-resistant steels such as 16Mo, 12CrMo, 15CrMo, etc., is generally around 0.5%.
When the molybdenum content in alloy steel is between 0.6% and 1.0%, molybdenum can reduce the plasticity and toughness of the alloy steel and increase its quenching tendency.
Vanadium can increase the strength of steel, refine grain size, reduce the tendency of grain growth, and improve hardenability.
Vanadium is a relatively strong carbide-forming element, and the carbides it forms are stable below 650℃.
It also has age-hardening effects. Vanadium carbides have high-temperature stability and can improve the high-temperature hardness of steel. Vanadium can also change the distribution of carbides in steel, but it is prone to forming refractory oxides, making welding and cutting difficult.
Generally, when the vanadium content in the weld metal is around 0.11%, it can play a role in nitrogen fixation, turning an unfavorable situation into a favorable one.