Have you ever wondered how to quickly distinguish between carbon steel and stainless steel? Understanding the differences is crucial in various applications, from construction to manufacturing. This article delves into the key methods of identifying these two types of steel, focusing on spark tests, color changes, and other distinguishing characteristics. By the end, you’ll have a clear understanding of how to effectively identify carbon steel and stainless steel, ensuring you choose the right material for your projects. Dive in to learn these essential techniques and enhance your material selection skills.

Does stainless steel spark when grinding?
Yes, stainless steel does indeed produce sparks during grinding operations. This phenomenon occurs due to the rapid heating of microscopic metal particles that are dislodged during the abrasive process. As the grinding wheel contacts the stainless steel surface, it generates significant friction, causing these tiny metal fragments to reach temperatures exceeding 1000°C (1832°F). At such high temperatures, the particles oxidize rapidly and emit visible light, manifesting as sparks.
The characteristics of these sparks—including color, intensity, and pattern—can vary depending on the specific grade and composition of the stainless steel being processed. For instance, austenitic stainless steels (e.g., 304, 316) typically produce shorter, more numerous sparks with a reddish-orange hue, while martensitic stainless steels (e.g., 420, 440C) often generate longer, brighter sparks with a yellowish tint. Factors such as the grinding wheel’s grit size, rotational speed, and applied pressure also influence the spark formation. It’s worth noting that while spark generation is common in grinding stainless steel, proper safety measures, including eye protection and spark containment, should always be implemented to prevent potential hazards in the workshop environment.
When steel is ground against an abrasive wheel under pressure, the material is reduced to fine particles through abrasive action. These particles are rapidly heated due to the mechanical friction and ejected by the centrifugal force of the rotating wheel.
As the heated steel particles come into contact with oxygen in the air, they undergo rapid oxidation. This exothermic reaction generates sufficient heat to bring the steel particles near their melting point, causing them to emit light. The trajectory of these luminous particles follows a streamlined path, creating the characteristic spark pattern.
The oxidation process begins with the formation of an iron oxide film (2Fe + O2 → 2FeO) on the particle surface. Simultaneously, within the particles, carbon present in the form of iron carbide (Fe3C) decomposes at high temperatures, releasing elemental carbon (Fe3C → Fe + C). This liberated carbon then reacts with the surface iron oxide, producing gaseous carbon monoxide.
This reaction creates a cyclic process where carbon atoms reduce the surface iron oxide, allowing it to react with atmospheric oxygen and re-oxidize. Concurrently, this triggers further reactions with internal carbon, leading to the accumulation of carbon monoxide gas within the particle.
When the internal gas pressure exceeds the surface tension of the particle’s outer layer, a micro-explosion occurs. This phenomenon manifests as a bright burst, resembling a miniature firework. If residual carbon remains within the resulting smaller particles, the oxidation-explosion cycle can repeat, potentially leading to second, third, or even fourth-generation bursts. This repetitive process creates the characteristic dendritic or branching pattern observed in spark testing.
The frequency and intensity of these explosions directly correlate with the carbon content of the steel. Higher carbon steels exhibit more frequent and pronounced bursts, resulting in more complex dendritic patterns with increased branching and “flower-like” formations. Conversely, lower carbon steels produce simpler spark patterns with fewer branches.
This relationship between carbon content and spark characteristics forms the basis of spark testing as a quick, qualitative method for identifying different steel grades in workshop settings. However, it’s important to note that while spark testing can provide valuable insights, it should be used in conjunction with other analytical methods for precise material identification and characterization.

Spark patterns, various parts and forms generally include:
When the test sample is ground on the grinding wheel, all sparks that are produced are collectively referred to as fire beams.
The fire beam can be divided into three main parts:
The fire beam that is nearest to the grinding wheel is called the root fire beam.
The middle part is called the middle fire beam.
The end part of the fire beam, which is farthest away from the grinding wheel, is called the tail fire beam. Refer to Fig. 12-1.
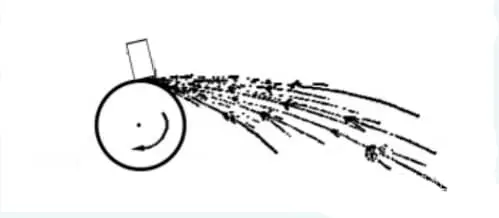
When grinding steel, grinding particles fly out at high speeds, creating bright lines known as streamlines.
Based on the shape characteristics of streamlines, there are three common forms: straight streamlines, wavy streamlines, and intermittent streamlines, as shown in Figure 12-2.

A burst pattern occurs in the middle of the streamline.
There are three common types of fireworks: dendritic fireworks, feathery fireworks, and bracts.
Branch-shaped fireworks resemble tree branches, with more or fewer branches, including two forks, three forks, and many forks.
There are different levels of splitting, including primary split, secondary split, and multiple split.
Feather-shaped explosions are a special form of rimmed steel explosion that resemble feathers. Bracts are special expansion forms that occur in the middle of the streamline and include bursting before and after the expansion part.
Please refer to Figure 12-3. If the bract flower appears at the end of the streamline, it is also referred to as the bract flower tail flower.

The point where the streamline bursts halfway is called a node.
Some fireworks have bright and plump nodes, while some fireworks have no obvious nodes.
When the spark bursts, the streamer is called the awn line.
Dendritic fireworks can be regarded as the collection form of most awn lines.
Sparks in the form of dots between the exploding awn lines or near the streamline.
Cauliflower is an abnormal form of streamline tail.
There are three kinds of common tail flowers: fox tail flower, spear tip tail flower and magnolia tail flower. As shown in Fig. 12-4.


Fig. 12-4 Tail flower form
The color and lightness of the whole flame beam or part of the spark.
The primary tool used for spark identification is a grinder.
Grinding machines can be either desktop or portable.
A bench grinder is suitable for inspecting steel samples and small-shaped parts.
A portable grinder can be used to identify batches of steel in workshops and warehouses.
The power of the motor used for a bench grinder is 0.5 KW, and the rotating speed is around 3000 RPM.
The motor for a portable grinder has a power of 0.2 KW and a speed of 2800 RPM.
Excessive power and speed can cause sparks to scatter, which is not conducive to identification.
If the power and speed are too low, it will be difficult to grind alloy tool steel and high-speed steel containing tungsten, and it may even fail to produce a flame beam.
The grinding wheel should have a grain size of 46# or 60# (preferably 60#) and medium hardness 200mm, and the thickness should be 20~25mm.
The grinding wheel for a portable grinding machine can have a diameter of 9020mm.
It is advisable not to frequently change the tools such as the grinding machine and grinding wheel granularity.
Having knowledge and familiarity with the tools’ performance is an essential aspect of identifying sparks.
The spark form can vary due to changes in wheel speed and particle size of different grinders.
The sharpness and roundness of the grinding wheel’s friction surface must be regularly maintained to ensure a consistent projection force.
If the grinding wheel is not sharp, it can reduce the streamline, while if the roundness is not maintained, steel may jump while rubbing against it. Therefore, the roundness of the grinding wheel should not be too small.
Before starting work, it’s important to identify a standard sample to correct for the potential influence of the objective environment.
The brightness of the work environment can significantly affect the observation of sparks.
The identification site should not be overly bright, but it does not need to be completely dark. It is important to maintain consistent brightness to ensure accurate identification.
Generally, it is not advisable to operate outdoors. However, if outdoor operation is necessary, a movable tarpaulin covered with black cloth should be used to avoid interference from strong light, such as that from rabbits.
A set of standard steel samples with known steel grades should be provided for comparison in learning and identification. The more comprehensive the steel samples, the better.
To determine the correct content of each element, the standard steel samples must undergo chemical analysis.
In the machinery manufacturing industry, accurate material selection and heat treatment are critical challenges faced by technical professionals. The proper identification and utilization of steel grades are paramount to ensure product quality, safety, and cost-effectiveness.
Incorrect material selection or inadvertent mixing of steel grades during manufacturing can lead to components failing to meet performance specifications, potentially resulting in significant economic losses or catastrophic failures. Therefore, a comprehensive understanding of steel varieties and their respective properties is essential for successful machinery production.
Steel identification methods can be broadly categorized into chemical and physical approaches. While chemical analysis offers high precision, it is primarily suitable for laboratory-based sampling inspections. The time and cost associated with chemical analysis make it impractical for on-site applications in most manufacturing environments.
Physical identification methods, though less precise than chemical analysis, prove invaluable for preliminary on-site assessments due to their practicality and the accumulated expertise of skilled technicians. Among these physical methods, spark identification and metallographic analysis stand out as the most efficient and widely applicable techniques.
Spark identification, in particular, has gained widespread adoption in the industry due to its numerous advantages:
The significance of spark identification becomes apparent in several critical stages of the manufacturing process:
Carbon is the main element of steel grade, and its activation form changes with the increase of carbon content.
Spark patterns of common steels are as follows:

Fig. 12-6 30 Steel
The flame beam appears entirely yellow, featuring a thick streamline in the middle, with slightly thinner ones at the root and slightly larger fireworks at the tail. Additionally, there are long awn streamlines that hang slightly.
In the case of the secondary explosion, it has multiple branches with bright explosion nodes.

Fig. 12-7 40 Steel
The length of the fireworks beam has slightly increased. All of the fireworks are now secondary bursts, and the fuse line is long and thick. Moreover, there are now more fireworks in the entire beam, and some pollen is starting to appear. The tail of the firework beam is also larger, and the color is a bright yellow.

Fig. 12-8 45 Steel
The length of the fire beam is longer than that of 40 steel. The shape of the fireworks is larger, and the number of streamlines and fireworks has increased. The streamlines are thicker, and the awn line is longer. There is a proper amount of pollen between the streamlines, and they emit forcefully, resulting in a larger degree of bursting. The nodes are bright, and the number of fireworks at the tail is significantly more than that of 40 steel. Additionally, the color is bright yellow.

Fig. 12-9 50 Steel
The length of the flame beam is equivalent to that of a 45 steel beam.
The explosion pattern is significant, with an increased number of streamlines and explosions. The streamlines are thick, with long awn lines and pollen between them, making the explosion powerful. The nodes are bright, and the number of explosions at the tail is noticeably higher than that of a 45 steel beam. The color of the flame is bright yellow.

The entire flame is yellow, with a slightly thick and long streamline and a straight shape. The middle portion towards the pouring tail is slightly drooping.
A single explosion pattern with multiple branches, made of carbon structural steel with the same carbon content, is slightly more regular than the explosion pattern. The degree of explosion is large, and the nodes are brighter.
The presence of chromium at this stage demonstrates its role in lengthening and cracking.

The spark beam is bright yellow, and it has many streamlined lines. The secondary burst of the compound flower is large, neat, and regular with a significant number of fireworks. The awn line is long and thick, and the flower angle is clear and well-separated.
There is an adequate amount of pollen, and the burst degree is high with thick streamline, slightly drooping from the middle to the tail. The burst degree of the big branch chrysanthemum is even more intense.
Currently, the medium carbon low inscription still serves the purpose of promoting the explosion.

Fig. 12-12 20CrMo Steel
The flame beam of the material is shorter than that of 20Cr steel. The streamline is slightly thinner, and there are multiple bifurcations and a single explosion at one time.
When compared with 20CrMo, the explosion pattern has shrunk, the degree of explosion is weakened, the nodes are not very bright, and the color is yellow. Additionally, the tail of the streamline has gun tip tail flowers.
Molybdenum has the property of inhibition at this stage.
Although chromium is an explosive element, it coexists with molybdenum, and its properties become subordinate.

Fig. 12-13 40CrMo Steel
The flame color of 42CrMo steel is slightly darker than that of 40Cr steel, and its streamline is similar. It forms secondary explosion compound flowers with an appropriate amount of pollen, and the nodes appear bright. However, the explosion patterns are irregular and messy, and the degree of explosion is slightly weakened. At the tail, there is a gun tip tail flower, which is not seen in 20CrMo steel.
From this, it can be inferred that the carbon content has a certain impact on molybdenum.

Fig. 12-14 60Si2Mn Steel
The fire beam has a moderate length and a reduced streamline, and is slightly thick. Most of them burst twice, while some burst three times with a small flower type and an obvious node of silicon bud. These types have few and short awn lines, a slightly weaker burst degree, and no pollen. The spark color and explosion node are not very bright.

Fig. 12-15 GCr15 Steel
The fire beam has a moderate length and features many streamlined and triple burst patterns. The streamlines are slightly thin, and they are densely covered with branch-shaped fireworks.
The amount of fireworks is large, the patterns are small, and the awn line is thin and irregular. There is a significant amount of pollen between the awn lines, and the nodes are not very distinct. The color of the fireworks is orange.
The internal organization is troostite pearlite in the hot-rolling state. The fire beam is long and thick, and it features three bursts. The burst intensity is strong, the awn line is long, and there is a significant amount of pollen between the awn lines. The burst nodes are bright, and the tail pattern is long and located in the middle.

Fig. 12-16 Cr12MoV Steel
The fire beam is thin and extremely short, with a wavy and intermittent streamline that appears to be numerous and slender.
The fireworks are exceptionally powerful, producing sparks that burst into three different flowers with multiple branches and significant stars. The flowers contain numerous broken flowers and pollen, and are full of fire.
The end of the streamline has an obvious gun-shaped tail flower as a result of the molybdenum content. Additionally, the streamline at the tail is slightly thicker, giving the material a hard feel when rubbed.
Color: yellow to orange. Spark form is not different from Cr12.

Fig. 12-17 5CrMnMo Steel
The fire beam is the thickest and longest, the streamline is medium in thickness, and the burst is the second strongest. All of them burst three times, sometimes with a few flowers, and there are molybdenum gun tip tail flowers.
The flower shape is a multi-branched three or four-segment star-shaped flower with a lancet tail flower. The awn line is dense, the distribution area of the flowers accounts for 55-60% of the whole fire bundle, the flower shape is large, and the flower angle is wide.
In terms of color, the fire beam is bright yellow, and the nodes are yellow to white. The resistance is less strong when grinding.

Fig. 12-18 3Cr2W8V Steel
The beam of fire is relatively long and the streamline is very thin, wavy, and intermittent. The burst is weak, with only a small amount of flowers in a bald fox-tail shape and size.
Body color: fuchsia.
Bald and solitary, light cherry red.
It feels very resistant when grinding.

Fig. 12-19 W6Mo5Cr4V2 Steel
The flame beam appears as a short, bright orange-yellow color, with a dark red hue at the base.
There are a few irregular streamlines along with some wave-like patterns.
The streamlines are not very thick and have a medium length.
The tail streamline is thicker and resembles a willow leaf with tail flowers, and the tip has a slight baldness.
The fireworks are few in number but have a large shape.
There are only a few awn lines, which are also bald.
The tail streamline droops downwards.
Judging the steel grade of the tested sample based on the observed spark pattern can be challenging.
This is because the spark patterns may exhibit subtle differences that are difficult to accurately describe and express. A skilled practitioner with ample experience and expertise is required to accurately discriminate between these subtle differences in spark patterns.
Currently, it is necessary to use spark identification only to confirm whether a material belongs to the intended steel grade.
When identifying a batch of parts, the first part should be carefully observed and analyzed. Once it has been confirmed that steel No. 1 is being used correctly, the part should be lightly ground to observe the basic characteristics of sparks when it is least worn.
This feature should be kept in mind, and the remaining parts can be ground with light pressure. This approach not only aids in identification but also minimizes wear and tear on the parts, thereby avoiding any negative impact on their appearance or functionality.
At this time, it is important to focus on the fundamental differences between the two steel grades in their spark patterns. Once you have a clear understanding of their respective characteristics and key distinctions, it becomes much easier to differentiate between them.
If the discriminator understands the basic use of steel and is familiar with the common sense of the materials that should be used to make various parts, it can be of great help in identifying sparks.
One factor to consider is whether dendritic explosion occurs when grinding the sparks. If there is dendritic explosion, it can be further inferred from the following situations:
① If dendritic explosion occurs normally, and there are no sparks in other special forms, it is mostly carbon steel (killed and semi-killed steel).
At this point, if the pattern is a split burst and the burst pot is relatively sparse, it indicates that the carbon content is low, and it belongs to the low carbon range of carbon steel.
If the pattern is a secondary, tertiary, or a small amount of multi-split dendritic explosion, the amount of explosion is medium, and the distance between the explosions is clear, indicating that the carbon content of the sample is about 0.4% C, and it belongs to the carbon steel in the medium carbon range.
If the explosion is a multi-forked tree-shaped explosion, the amount of explosion is large, and the distance between the explosions is small, indicating that the carbon content is high and it belongs to high carbon steel. When the explosion is crowded, it confirms that the carbon content is high.
② If the explosion exhibits a dendritic pattern and has a feathery appearance, it indicates that the steel is rimmed with very low silicon content. The carbon content can be estimated roughly based on the explosion amount, which can help infer the steel grade.
③ To roughly identify the type of steel: