What if you could unlock the hidden strength in metals with just a few ingenious techniques? This article delves into four proven processes—solid solution strengthening, work hardening, fine grain strengthening, and second phase strengthening—that significantly enhance metal durability and performance. Discover how these methods transform ordinary metals into robust, high-performance materials, and learn the science behind their applications. Dive in to understand how these processes can benefit your next engineering project.
The phenomenon of solid solution of alloy elements in the matrix metal causing a certain degree of lattice distortion improves the strength of the alloy.
The atoms of the solute that are dissolved in the solid solution cause lattice distortion, which increases resistance to dislocation movement and makes slipping more difficult. As a result, the strength and hardness of the solid solution of the alloy are increased. This phenomenon of metal strengthening through the formation of a solid solution by dissolving a specific solute element is known as solid solution strengthening.
When the concentration of the solute atom is optimal, the material’s strength and hardness can be improved, but its toughness and plasticity decrease.
The greater the concentration of solute atoms, the more pronounced the strengthening effect, particularly at low concentrations, where the effect is more significant.
The greater the difference in atomic size between the solute atom and the matrix metal, the stronger the strengthening effect.
Interstitial solute atoms have a stronger solid solution strengthening effect than replacement atoms. Additionally, the lattice distortion caused by interstitial atoms in body-centered cubic crystals is asymmetric, resulting in a stronger strengthening effect than that in face-centered cubic crystals. However, the solid solubility of interstitial atoms is very limited, so the actual strengthening effect is also limited.
The greater the difference in the number of valence electrons between the solute atom and the matrix metal, the more pronounced the solid solution strengthening effect becomes. In other words, the yield strength of the solid solution increases as the concentration of valence electrons increases.
The degree of solid solution strengthening mainly depends on the following factors:
(1) The size difference between matrix atoms and solute atoms:
The larger the size difference, the more the original crystal structure is disturbed, and the more challenging it becomes for dislocations to slip.
(2) The amount of alloy elements:
The greater the amount of alloy elements added, the stronger the strengthening effect.
If too many atoms that are either too large or too small are added, the solubility will be exceeded. This results in another strengthening mechanism known as dispersion phase strengthening.
(3) The solid solution strengthening effect of interstitial solute atoms is greater than that of replacement atoms.
(4) The greater the difference in the number of valence electrons between the solute atom and the matrix metal, the more pronounced the solid solution strengthening effect becomes.
The yield strength, tensile strength, and hardness are stronger in comparison to those of pure metal.
In most cases, the ductility is lower compared to that of pure metal.
The electrical conductivity is significantly lower compared to that of pure metal.
Solid solution strengthening can improve resistance to creep, or the loss of strength at high temperatures.
With the increase of cold deformation, the strength and hardness of metal materials increase, but the plasticity and toughness decrease.
The phenomenon of increased strength and hardness of metal materials, accompanied by a decrease in plasticity and toughness during plastic deformation below the recrystallization temperature, is known as cold work hardening.
The reason for this is that during the plastic deformation of the metal, grain slipping occurs and dislocations become entangled, causing the grain to elongate, break, and fibrose, resulting in residual stress within the metal.
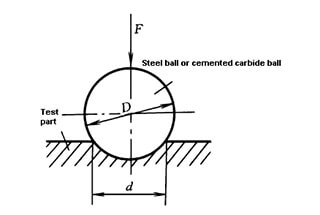
The degree of work hardening is commonly expressed as the ratio of the microhardness of the surface layer after processing to that before processing, and the depth of the hardened layer.
From the perspective of dislocation theory:
(1) The intersection of dislocations impedes their movement through the formation of cut-off dislocations;
(2) The reaction between dislocations creates fixed dislocations that further impede their movement;
(3) Dislocation proliferation leads to an increase in dislocation density, further increasing the resistance to dislocation movement.
Work hardening makes further processing of metal parts challenging.
For instance, during cold rolling, the steel plate will become increasingly harder to the point where it can no longer be rolled. Therefore, it is necessary to include intermediate annealing in the processing process to eliminate the work hardening through heating.
For example, in the cutting process, the surface of the workpiece becomes brittle and hard, causing accelerated tool wear, increased cutting force, and so on.
Work hardening can improve the strength, hardness, and wear resistance of metals, particularly for those pure metals and some alloys that cannot be strengthened through heat treatment.
Examples include cold-drawn high-strength steel wire and cold-coiled springs, which use cold working deformation to enhance their strength and elastic limit.
For instance, the crawler of tanks and tractors, the jaw plate of crushers, and the turnout of railway tracks also use work hardening to improve their hardness and wear resistance.
The surface strength of metal materials, parts, and components can be significantly improved through cold drawing, rolling, and shot peening (as described in surface strengthening).
When parts are subjected to stress, local stress in some areas can often exceed the material’s yield limit, leading to plastic deformation. However, work hardening restricts the continued development of plastic deformation, thereby improving the safety of parts and components.
When a metal part or component is stamped, plastic deformation is accompanied by strengthening, resulting in the transfer of deformation to the surrounding unworked, hardened part.
Through repeated alternating actions, cold-stamped parts with uniform cross-section deformation can be obtained, and the cutting performance of low carbon steel can be improved, making it easier to separate chips.
However, work hardening also makes further processing of metal parts challenging. For example, cold-drawn steel wire becomes difficult to draw further due to work hardening, requiring a significant amount of energy, and may even break. As a result, it must be annealed to eliminate the work hardening before being drawn further.
Similarly, in the cutting process, making the surface of the workpiece brittle and hard through work hardening increases cutting force and accelerates tool wear during re-cutting.
The method of enhancing the mechanical properties of metal materials through grain refinement is known as fine grain strengthening.
In industry, refining grains is utilized to improve the strength of materials.
Metals are usually composed of many grains and are referred to as polycrystals. The size of the grains can be expressed in terms of the number of grains per unit volume, with a greater number indicating finer grains.
Experiments show that fine-grained metals have higher strength, hardness, plasticity, and toughness compared to coarse-grained metals at room temperature. This is because the plastic deformation caused by external forces in fine grains can be dispersed across more grains, leading to more uniform plastic deformation and reduced stress concentration.
Furthermore, the finer the grain, the larger the grain boundary area and the more tortuous the grain boundary becomes, making it more difficult for cracks to propagate.
Therefore, the method of enhancing material strength through grain refinement is referred to as fine grain strengthening in industry.
The finer the grain, the fewer dislocations (n) present in the dislocation cluster, resulting in lower stress concentration and increased material strength.
The fine grain strengthening strengthening law states that the more grain boundaries present, the finer the grains.
According to the Hall-Petch relation, the smaller the average grain size (d), the higher the material’s yield strength.
Methods for refining the grains of cold-deformed metals include:
The grain size can be controlled by adjusting the degree of deformation and annealing temperature.
In comparison to single-phase alloys, multiphase alloys contain a second phase in addition to the matrix phase.
When the second phase is uniformly dispersed as fine particles within the matrix phase, it results in a significant strengthening effect, referred to as second phase strengthening.
The second phase contained in the alloy has the following two effects on the movement of dislocations:
(1) Strengthening effect of non-deformable particles (bypass mechanism).
(2) Strengthening effect of deformable particles (cutting mechanism).
Both dispersion strengthening and precipitation strengthening are special cases of second phase strengthening.
The second phase strengthening is primarily due to the interaction between the second phase and dislocations, which impedes dislocation movement and enhances the alloy’s resistance to deformation.
The strength of metal materials is primarily affected by its composition, microstructure, and surface state.
The second factor is the stress state, such as the rate of applied force and the mode of loading, which can result in different strengths, for example, the tensile strength of ultra-high strength steel may decrease when tested in a hydrogen atmosphere.
The geometric shape and size of the sample and the test medium also have a significant impact and can sometimes be decisive.
There are only two ways to strengthen metal materials:
In engineering materials, the strength is generally improved through a comprehensive strengthening effect to achieve better overall properties.