Have you ever wondered how the carbon content in metals is precisely measured? This article explores various methods, from infrared absorption to electrochemical analysis, shedding light on their principles and applications. By the end, you’ll understand the strengths and limitations of each technique, helping you appreciate the science behind metal quality control.
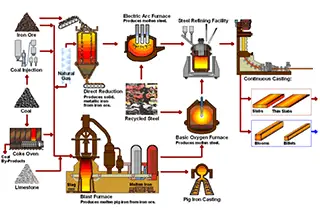
The development and utilization of metals and their composite materials often necessitate precise control and accurate determination of their carbon and sulfur content. These elements significantly influence the mechanical properties, microstructure, and overall performance of metallic materials.
Carbon in metal materials exists in various forms, including free carbon (graphite), interstitial solid solution carbon, carbides, dissolved gaseous carbon, surface carburization layers, and organic carbon coatings. Each form contributes differently to the material’s characteristics and requires specific consideration during analysis.
Currently, several methods are employed for analyzing carbon content in metals, including:
However, each measurement method has a limited scope of application, and the measurement results can be significantly influenced by various factors. These include the specific form of carbon present, the efficiency of carbon release during oxidation or excitation, sample preparation techniques, and the inherent instrument blank value. Consequently, the same analytical method may yield different levels of accuracy and precision in different scenarios or for different material compositions.
This article provides a comprehensive overview of the current analysis methods, sample preparation techniques, instrumentation, and application fields for carbon analysis in metals. It aims to guide materials scientists, metallurgists, and quality control professionals in selecting the most appropriate analytical approach for their specific needs, ensuring accurate carbon content determination across a wide range of metallic materials and industrial applications.
The combustion infrared absorption method, which is based on the infrared absorption method, is a specialized method for the quantitative analysis of carbon (and sulfur).
The principle behind this method involves burning the sample in an oxygen stream to produce CO2.
At a specific pressure, the energy absorbed by the CO2 from infrared rays is proportional to its concentration.
Therefore, the carbon content can be calculated by measuring the change in energy before and after the CO2 gas passes through the infrared absorber.
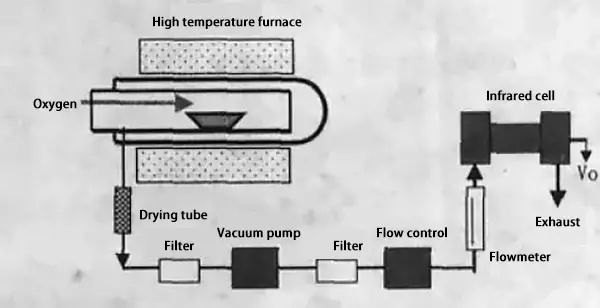
Principle of combustion-infrared absorption method
In recent years, infrared gas analysis technology has advanced rapidly, leading to the rapid emergence of various analytical instruments based on the principles of high-frequency induction heating, combustion, and infrared spectrum absorption.
When determining carbon and sulfur content using the high-frequency combustion infrared absorption method, the following factors should generally be taken into consideration: sample dryness, electromagnetic sensitivity, geometric size, sample size, type of flux, proportion, order of addition, amount of addition, blank value settings, etc.
This method has the advantage of providing accurate quantification with minimal interference.
It is suitable for users who place a high emphasis on the accuracy of carbon content and have sufficient time for testing during production.
When an element is energized by heat or electricity, it transitions from its ground state to an excited state, which will then spontaneously return to the ground state.
During the process of returning from the excited state to the ground state, each element releases its characteristic spectrum, and its content can be determined based on the intensity of this spectrum.
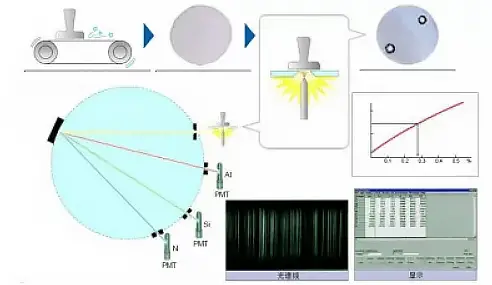
Principle of Emission Spectrometer
In the metallurgical industry, where production demands are high, it is necessary to quickly analyze the content of all major elements in the furnace water, not just carbon.
Spark direct reading emission spectrometers have become the preferred choice in this industry due to their ability to provide quick and stable results.
However, this method has specific requirements for sample preparation.
For instance, when analyzing cast iron samples using spark spectroscopy, it is necessary that the carbon on the surface of the sample be in the form of carbides, and free of graphite, otherwise, the analysis results may be affected.
Some users take advantage of the fast cooling and whitening properties of thin slices to determine the carbon content in castings by spark spectroscopy, after making the samples into slices.
When using spark spectroscopy to analyze carbon steel wire samples, the samples must be processed strictly, and placed either “upright” or “flat” on the spark table for analysis using a small sample analysis fixture, in order to improve the precision of the analysis.
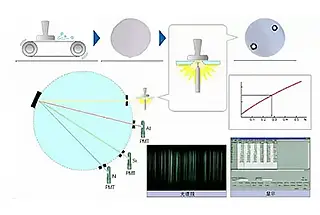
The Wavelength Dispersive X-ray Analyzer can quickly and simultaneously determine the content of multiple elements.

Principle of Wavelength Dispersive X-ray Fluorescence Spectrometer
The wavelength dispersive X-ray fluorescence spectrometer (WDXRF) utilizes X-ray excitation to cause the inner electrons of the element atoms to transition in energy level and emit secondary X-rays, also known as X-ray fluorescence.
The spectrometer splits the light using a crystal, and the detector detects the diffracted characteristic X-ray signal.
By synchronizing and continuously changing the diffraction angle of the spectroscopic crystal and the controller, the characteristic X-ray wavelength and intensity of each wavelength X-ray produced by various elements in the sample can be obtained for qualitative and quantitative analysis.
First produced in the 1950s, WDXRF has been widely used in the geology department due to its ability to simultaneously determine multiple components in complex systems, leading to improved analysis speed.
However, the low fluorescence yield of light element carbon and significant absorption and attenuation of its characteristic radiation by heavy matrix materials such as steel present challenges in XRF analysis of carbon.
Repeated measurements of the ground surface may also result in increasing carbon content values. As a result, this method is not as widely used as the previous two methods mentioned.
The non-aqueous titration method is a method of titration that uses a non-aqueous solvent. This method allows for the titration of weak acids and bases that cannot be titrated in an aqueous solution by selecting an appropriate solvent to increase their acidity or alkalinity.
For example, the weakly acidic carbonic acid generated by CO2 in water can be accurately titrated by using different organic reagents.
The commonly used non-aqueous titration method includes the following steps:
This method is toxic, long-term exposure can affect human health, and is difficult to operate, especially when the carbon content is high and the solution must be preset. Any carelessness can cause low results because of the escape of carbon.
Additionally, the reagents used in the non-aqueous titration method are mostly flammable products, and high-temperature heating operations are involved in the experiment, so the operators must have a strong awareness of safety.
The flame atomization detector is used in conjunction with gas chromatography to heat the sample in hydrogen and then detect the emitted gases, such as CH4 and CO, using the flame atomization detector-gas chromatography.
This method is ideal for those who have extremely low carbon content and high requirements for their test results. For instance, some users have used this method to test for trace amounts of carbon in high-purity iron, with a content of 4 µg/g and an analysis time of 50 minutes.
Some users have introduced the use of potentiometric analysis to determine low carbon content in alloys.
After the iron sample is oxidized in an induction furnace, a potassium carbonate solid electrolyte-based electrochemical concentration cell is used to analyze and determine the gaseous product to determine the carbon concentration.
This method is especially suitable for the determination of very low concentrations of carbon. The accuracy and sensitivity of the analysis can be controlled by adjusting the composition of the reference gas and the oxidation rate of the sample.
However, this method has limited practical applications and remains mostly in the experimental research stage.
When refining steel, it’s often necessary to monitor the carbon content of the molten steel in real-time in a vacuum furnace.
Some scholars in the metallurgical industry have introduced methods of using waste gas information to estimate the carbon concentration.
By using the oxygen consumption and concentration during the vacuum decarburization process, along with the flow rates of oxygen and argon, they estimate the carbon content in the molten steel.
Additionally, some users have developed methods and instruments for rapid determination of trace amounts of carbon in molten steel. By blowing a carrier gas into the molten steel, they estimate the carbon content by measuring the oxidized carbon in the carrier gas.
These online analysis methods are useful for quality management and performance control in the steel production process.