What if the key to creating the perfect metal powder lay in the methods used to produce it? This article dives into various metal powder preparation techniques, from reduction and electrolysis to atomization and mechanical pulverization. Readers will discover how these methods impact the quality and characteristics of metal powders, essential for applications in metallurgy, electronics, and beyond. By the end, you’ll understand the principles behind these methods and how they shape the future of metal powder technology.

The preparation of metal and its compound powders has developed numerous methods, and several classifications for these methods have been established.
Depending on the state of the raw material, methods can be divided into solid, liquid, and gas methods; based on the state of reactants, they can be categorized as wet and dry methods; and according to the production principle, they can be split into physicochemical and mechanical methods.

Generally, in physicochemical methods, the most important are reduction, reduction-combination, and electrolysis, while in mechanical methods, atomization and mechanical milling are most prominent.
The choice of metal powder production method depends on the raw material, powder type, performance requirements of the powder material, and the efficiency of powder production.
As the application of powder metallurgy products becomes increasingly widespread, the requirements for the size, shape, and performance of powder particles are becoming higher and higher.
Therefore, powder preparation technology is continuously evolving and innovating to meet the requirements for particle size and performance.
2.1.1 Reduction Method
The reduction of metal oxides and salts is a widely used method for powder preparation. Solid carbon can be used to reduce iron and tungsten powder, while hydrogen or decomposed ammonia is used to produce tungsten, molybdenum, iron, copper, cobalt, nickel powders.
Iron powder can also be produced using converted natural gas and coal gas. Sodium, calcium, magnesium, and other metals can act as reducing agents to produce tantalum, niobium, titanium, zirconium, thorium, uranium, and other rare metal powders.
The basic principle of this reduction method is that the affinity of the utilized reducing agent for oxygen is greater than that of the metal in the oxide or salt, thus enabling the reduction of the metal by capturing the oxygen in the metal oxide or salt.
Since different metal elements react differently with oxygen, the stability of the resulting oxides also varies. The degree of stability of the oxide can be characterized by the size of ΔG during the oxidation process. The smaller the ΔG value during the reaction, the higher the stability of the oxide, indicating a greater affinity for oxygen.
The advantages of this method include its simplicity, easy control of process parameters, high production efficiency, and low cost, making it suitable for industrial production.
However, it is only applicable to metal materials that readily react with hydrogen and become brittle and prone to fracture after hydrogen absorption.
2.1.2 Metal Thermal Reduction and Reduction-Combination Method
Metal thermal reduction involves reducing raw materials that can be solid, gaseous, or even molten salts, with the latter two having the characteristics of gas phase reduction and liquid phase precipitation.
Common industrial applications of the metal thermal reduction method include the use of calcium to reduce TiO2, ThO2, UO2, and others; magnesium to reduce TiCl4, ZrCl4, TaCl5, and others; sodium to reduce TiCl4, ZrCl4, K2ZrF6, K2TaF7, and others; and calcium hydride (CaH2) to co-reduce chromium oxide and nickel oxide for the production of nickel-chromium stainless steel powder.
The reduction-combination method refers to the process of obtaining carbides and borides through the reaction of carbon, boron carbide, silicon, nitrogen, and refractory metal oxides.
2.1.3 Electrolysis Method
The electrolysis method involves the deposition of metal powder at the cathode via the electrolysis of molten salts or their aqueous solutions. Almost all metal powders can be produced by electrolysis, with copper, silver, and tin powders being particularly suitable.
Electrolysis can be further divided into aqueous solution electrolysis, organic electrolyte electrolysis, molten salt electrolysis, and liquid metal cathode electrolysis.
The advantage of this method is that it produces metal powder with high purity, usually with a purity of 99.7% or more for single-element powders. Additionally, electrolysis can precisely control the particle size, enabling the production of ultra-fine powders.
However, the electrolysis method consumes a large amount of electricity, resulting in higher powder production costs. Aqueous electrolysis can produce Cu, Ni, Fe, Ag, Sn, Fe-Ni and other metal (alloy) powders, while molten salt electrolysis can produce Zr, Ta, Ti, Nb, and other metal powders.
2.1.4 Hydroxyl Method
Certain metals (such as iron, nickel, etc.) are synthesized with carbon monoxide to form metal carbonyl compounds, which are then thermally decomposed into metal powder and carbon monoxide.
The resulting powder is extremely fine and pure, but the process is costly. Industrially, it’s primarily used to produce fine and ultrafine powders of nickel and iron, as well as alloy powders of Fe-Ni, Fe-Co, Ni-Co, and others.
2.1.5 Chemical Displacement Method
The chemical displacement method is based on the reactivity of metals, where a more reactive metal displaces a less active metal from its salt solution, producing a metal (metal powder particles) that is further refined using other methods.
This method is mainly used in the preparation of powders from less active metals such as Cu, Ag, Au.
2.2.1 Atomization Method
The atomization method is a mechanical powder-making method, involving the direct pulverization of liquid metal or alloy to produce powder. It’s widely applied and is second only to the reduction method in scale.
Also known as the spray method, it can be used to produce powders of metals such as lead, tin, aluminum, copper, nickel, and iron. It can also be utilized in the production of alloy powders such as bronze, brass, carbon steel, and alloy steel.
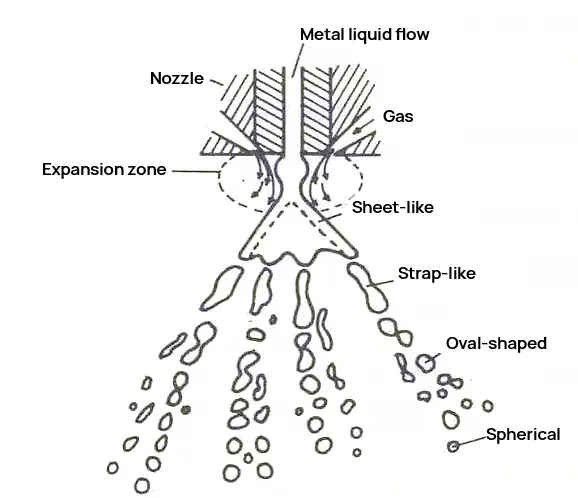
Atomization generally involves the use of high-pressure gas, high-pressure liquid, or high-speed rotating blades to break high-temperature, high-pressure molten metal or alloy into tiny droplets. These droplets then condense inside a collector to form ultrafine metal powder, a process that does not involve chemical changes.
Atomization is one of the main methods for producing metal and alloy powders. There are many atomization methods, such as dual-stream atomization, centrifugal atomization, multi-stage atomization, ultrasonic atomization technology, tightly coupled atomization technology, high-pressure gas atomization, laminar flow atomization, ultrasonic tightly coupled atomization, and hot gas atomization.
Atomized powder has advantages like a high degree of sphericity, controllable powder granularity, low oxygen content, low production cost, and adaptability to the production of various metal powders.
It has become the primary development direction for the preparation technology of high-performance and special alloy powders. However, atomization has disadvantages such as low production efficiency, a low yield rate of ultrafine powder, and relatively high energy consumption.
2.2.2 Mechanical Pulverization Method
Mechanical pulverization of solid metals is a distinct method for powder production, closely associated with the final state of solid strain and the formation and extension of cracks during pulverization.
Additionally, it serves as an indispensable complementary process for some powder production methods, such as grinding electrolytically produced brittle cathode precipitates, or grinding sponge-like metal chunks produced through reduction. Therefore, the mechanical pulverization method occupies a significant position in powder production.
The pulverization method varies according to the nature of the materials and the required degree of pulverization.
Depending on the mode of external force application, material pulverization generally occurs through compression, impact, grinding, and focused splitting. The operational principles of various pulverization equipment are based on these methods.
Among these, ball milling primarily involves rolling ball and vibrating ball milling methods. This method utilizes the mechanism where metal particles break down into finer substances due to deformation at different strain rates.
Its advantages include low selectivity towards materials, continuous operation, high production efficiency, and it is suitable for dry and wet milling, facilitating the preparation of various metal and alloy powders. The downside is that classification is relatively difficult during the powder preparation process.
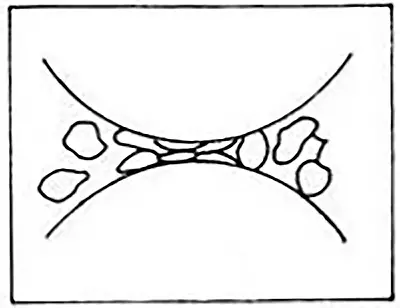
2.2.3 Grinding Method
The grinding method involves directing compressed gas through a specialized nozzle to the grinding area, causing the materials within this zone to collide and grind into powder.
The expanded airflow ascends along with the materials into the classification zone, where a turbine-type classifier separates the materials that have reached the desired granularity.
The remaining coarse powder returns to the grinding area for further grinding until it achieves the required granularity for separation. The grinding method, being a dry process, eliminates the need for material dehydration and drying operations.
The resulting product is of high purity, great activity, and good dispersibility, with fine granularity and a narrow distribution range. The particles have smooth surfaces and are extensively applied in industries such as non-metals, chemical raw materials, pigments, abrasives, health pharmaceuticals, and others for ultra-fine crushing.
However, the grinding method has some drawbacks, such as high equipment manufacturing costs, and in the production process of metal powder, a continuous supply of inert or nitrogen gas is required as the compressed gas source, which leads to substantial gas consumption.
Thus, it is only suitable for brittle metals and alloys’ crushing and powdering processes.
With the advancement of technology, metal powders have been developed and applied in fields such as metallurgy, chemical engineering, electronics, magnetic materials, fine ceramics, and sensors, displaying promising application prospects.
Metal powders are trending towards higher purity and superfine (nano) development. Although there are various methods for preparing ultrafine metal powders, each method has its limitations and there are many problems that need to be resolved and perfected.
Currently, the most widely used methods for producing metal powders are reduction, electrolysis, and atomization.
In addition, improvements on traditional production processes have led to many new production techniques and methods, such as ultrasonic atomization, rotating disk atomization, twin-roll and triple-roll atomization, multi-stage atomization, plasma rotating electrode process, and electric arc method.
Among the methods for producing metal powders, while many have been put into practical use, two main problems still exist: the scale is small and the production cost is high.
To promote the development and application of metal powder materials, it is necessary to make comprehensive use of different methods, to leverage their strengths and compensate for their weaknesses, and develop processes that yield larger production volumes and lower costs.