What makes metals so versatile and indispensable in engineering? This article explores the essential basics of metal crystallization, structure, and transformations. Discover how metals transition from liquid to solid states, the importance of crystal defects, and the fascinating phenomena of supercooling. By understanding these principles, you’ll gain insights into metal properties and their applications in various industries.
Metals are solid-state crystals.
The crystal structure is related to the properties, plastic deformation, and heat treatment phase transformations of metals.
The three most common lattices in metals are the body-centered cubic lattice, face-centered cubic lattice, and closely packed hexagonal lattice.
Crystal defects can be classified into three categories based on their geometric shapes: point defects, line defects, and plane defects.
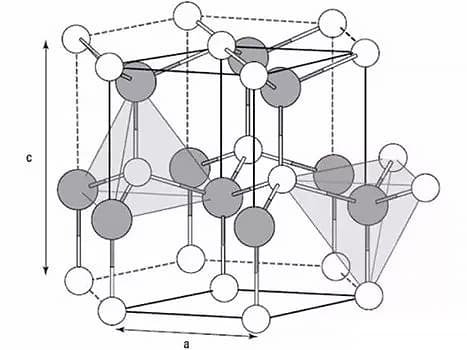
The process by which a metal transitions from a liquid to a solid (crystalline) state is known as metal crystallization.
(1) Cooling Curve and Supercooling Phenomenon
The cooling curve is a graph that displays the relationship between temperature and time during the cooling process of a material. The cooling curve of a metal crystal can be determined using thermal analysis methods. The process involves melting the metal to achieve as uniform a temperature as possible, cooling it at a set speed, recording the temperature changes over time, and plotting the data on a temperature-time graph to obtain the cooling curve, as shown in Figure 1.
The latent heat of crystallization that is released during crystallization counteracts the heat loss from the metal to the outside, causing a horizontal line to appear on the cooling curve. The temperature corresponding to this line is the actual crystallization temperature of the metal.
Experiments show that the actual crystallization temperature (T1) of the metal is always lower than the theoretical crystallization temperature (T0). This phenomenon is referred to as supercooling. Supercooling is a requirement for crystallization to occur. The difference between T0 and T1, △T = T0 – T1, is known as the supercooling degree.
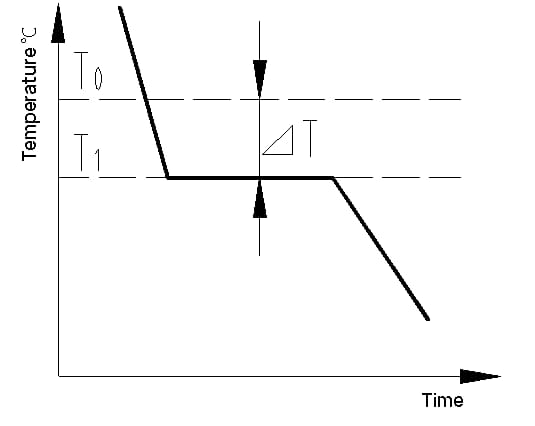
Fig. 1 cooling curve of pure iron crystal
(2) Crystallization Process
The process of crystallization involves the creation and expansion of nuclei. This process is known as nucleation and growth.
The phenomenon in which a metal transforms from one lattice structure to another as the temperature changes in the solid state is known as an isomorphic transformation.
Some metals that exhibit this transformation include iron, cobalt, titanium, tin, and manganese.
Crystals of the same metal element that exist in different lattice forms are referred to as allotropic crystals of the metal.
Phase: Refers to the uniform components within an alloy (or pure metal) that possess the same composition, structure, and properties, and are separated from one another by an interface.
The phase structure in alloys can be divided into two types based on the interaction between the constituent elements: solid solution and metal compound.
(1) Solid Solution: When the liquid alloy solidifies, the elements can still dissolve into each other to form a phase in which atoms of one element are dispersed throughout the lattice of another element. This phase is known as a solid solution.
(2) Metal Compound.
The alloy phase diagram, also known as the alloy equilibrium diagram or the alloy state diagram, is a diagram that illustrates the relationship between the temperature, composition, and state of an alloy under equilibrium conditions. It depicts the structural change laws of alloys with varying compositions as they are slowly cooled or heated to infinity.
The alloy phase diagram is an important tool for selecting the right alloy composition, analyzing the microstructure of the alloy, studying its properties, and determining casting, forging, and heat treatment processes.
(1) Homogeneous Phase Diagram: This type of diagram represents an alloy system where two components can be infinitely miscible in both the liquid and solid states. During solidification, the alloy forms a solid solution from the liquid phase, a process known as homogeneous transformation.
(2) Eutectic Phase Diagram: In this diagram, two components are completely miscible in the liquid state and have a eutectic transformation. Eutectic Transformation refers to the simultaneous crystallization of two solid phases with a specific composition from a uniform liquid phase with a specific composition at a particular temperature.
(3) Peritectic Phase Diagram: In this diagram, two components are infinitely miscible in the liquid state and form a finite solid solution in the solid state. There is also a state of peritectic transformation. Peritectic Transformation refers to the reaction between the liquid phase of a certain component and the solid phase of another component, which results in the formation of a new solid phase at constant temperature.

Steel is an iron-carbon alloy with a specific composition range.
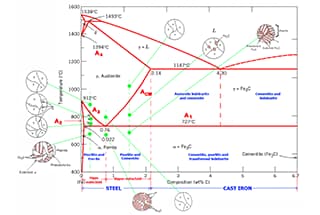
The iron-carbon alloy phase diagram illustrates the various equilibrium structures of iron-carbon alloys with varying compositions at different temperatures, as depicted in the Fe-Fe3C phase diagram.
From the Fe-Fe3C phase diagram, we can determine the temperature at which the phase transformation occurs in an iron-carbon alloy of a certain composition, also known as the critical point.
By analyzing the Fe-Fe3C phase diagram, it is possible to predict the phase transformation process in different temperature regions and the potential equilibrium structure upon cooling to room temperature.
Refer to the characteristic points in the Fe-Fe3C phase diagram for a description of each point in the iron-carbon alloy phase diagram and to the characteristic lines for a description of each line.
According to the iron-carbon alloy phase diagram, carbon steel with carbon content less than 2.11% and cast iron with carbon content greater than 2.11% are differentiated.
Based on structural characteristics, the iron-carbon alloy is divided into seven categories based on the carbon content in the iron-carbon alloy phase diagram:
(1) Industrial pure iron, with carbon content < 0.0218%;
(2) Eutectoid steel, with carbon content 0.77%;
(3) Hypoeutectoid steel, with carbon content ranging from 0.0218% to 0.77%;
(4) Hypereutectoid steel, with carbon content ranging from 0.77% to 2.11%;
(5) Eutectic white cast iron, with carbon content 4.30%;
(6) Sub-crystalline white cast iron, with carbon content ranging from 2.11% to 4.30%;
(7) Super-crystalline white cast iron, with carbon content ranging from 4.30% to 6.69%.
Metal: A material with good thermal conductivity and electrical conductivity, characterized by its opaque appearance and metallic luster. The conductivity of metals decreases as temperature increases, and they are known for their ductility and expandability.
A metal crystal is a solid in which atoms are arranged in a regular pattern.
Alloy: A substance with metallic properties composed of two or more elements, including metals and non-metals.
Solid Solution Strengthening: This occurs when solute atoms occupy the spaces or interstices of the solvent lattice, causing the lattice to become distorted and increasing the hardness and strength of the solid solution.
Compound: A new crystalline solid structure with metallic properties is formed by the combination of alloy components.
Mechanical Mixture: An alloy composition composed of two distinct crystal structures, even though it appears as a single entity with independent mechanical properties.
Ferrite: An interstitial solid solution of carbon in alpha-Fe (body centered cubic iron).
Austenite: An interstitial solid solution of carbon in gamma-Fe (face centered cubic iron).
Cementite: A stable compound (Fe3C) formed by the combination of carbon and iron.
Pearlite: A mechanical mixture composed of ferrite and cementite (F + Fe3C, containing 0.8% carbon).
Ledeburite: A mechanical mixture composed of cementite and austenite (containing 4.3% carbon).

Heat treatment of metals is a crucial process in mechanical manufacturing. Unlike other processing methods, heat treatment does not alter the shape or overall chemical composition of the workpiece, but instead enhances its performance by modifying its microstructure or surface chemical composition.
The purpose of heat treatment is to improve the internal quality of the workpiece, which is often not visible to the naked eye. To achieve the desired mechanical, physical, and chemical properties of a metal workpiece, heat treatment is often necessary in addition to the appropriate selection of materials and various forming processes.
Steel is the most widely used material in the mechanical industry, and its microstructure can be controlled through heat treatment. As a result, heat treatment of steel is a major aspect of metal heat treatment.
In addition to steel, heat treatment can also be used to modify the mechanical, physical, and chemical properties of aluminum, copper, magnesium, titanium, and their alloys, allowing for the attainment of various service properties.