Have you ever wondered how a simple element like nitrogen can transform steel’s properties? This blog explores nitrogen’s profound impact on steel’s microstructure, mechanical strength, and more. Discover how nitrogen can enhance steel’s durability and performance, making it indispensable in various applications. Prepare to uncover the fascinating role of nitrogen in revolutionizing steel technology!

① Nitrogen, analogous to carbon, forms an interstitial solid solution in iron, occupying octahedral sites in the crystal lattice. This dissolution significantly influences the steel’s mechanical properties and microstructure.
② Nitrogen is a potent austenite stabilizer, expanding the austenite phase field in the iron-carbon phase diagram. Its austenite-promoting effect is approximately 20-30 times stronger than that of nickel, making it an economical alternative in austenitic stainless steels. This property allows for reduced nickel content without compromising austenite stability, leading to cost-effective alloy designs.
③ When nitrogen diffuses into the steel surface, it can form highly stable nitrides with alloying elements such as chromium, aluminum, vanadium, and titanium. These nitrides, including CrN, AlN, VN, and TiN, significantly enhance surface hardness, wear resistance, and fatigue strength. The nitride formation process, known as nitriding, is a crucial surface engineering technique in the heat treatment of steels.
④ In high-chromium and high-chromium-nickel steels, nitrogen contributes to grain refinement and solid solution strengthening. This results in a more compact and robust microstructure, improving the steel’s overall strength, toughness, and corrosion resistance. The fine-grained structure also enhances the steel’s resistance to intergranular corrosion.
⑤ While nitrogen offers numerous benefits, excessive residual nitrogen content in steel can lead to detrimental effects. High nitrogen levels can cause the formation of gas porosity or blowholes during solidification, compromising the steel’s structural integrity. This phenomenon, known as nitrogen porosity, is particularly problematic in welding high-nitrogen steels and must be carefully controlled through proper melting and solidification practices.

① Nitrogen exhibits a potent solid solution strengthening effect in steel, significantly enhancing its hardenability. This occurs due to nitrogen atoms’ ability to distort the iron lattice, impeding dislocation movement and thereby increasing strength.
② In nitrogen-containing ferritic steels, a complex precipitation hardening mechanism can be observed. During tempering or prolonged exposure to room temperature following rapid cooling, ultra-fine nitrides (typically Fe16N2 or CrN) precipitate. These nanoscale particles act as obstacles to dislocation motion, further increasing the steel’s strength and hardness.
Nitrogen’s presence can induce strain aging in low-carbon steels, a phenomenon characterized by an increase in yield strength and decrease in ductility over time. This effect is particularly pronounced in steels with carbon content below 0.15%.
As the strength and hardness of steel increase due to nitrogen addition, there’s a corresponding decrease in toughness and an increase in notch sensitivity. This trade-off is crucial in engineering applications where both strength and fracture resistance are required.
The embrittling effect of nitrogen in steel is comparable to, and in many cases more severe than, that of phosphorus. Nitrogen’s potent impact on grain boundary cohesion and dislocation dynamics contributes to this pronounced embrittlement.
Nitrogen plays a significant role in blue brittleness, a phenomenon observed when steel is heated to 200-300°C (392-572°F). This temperature range allows nitrogen to rapidly diffuse to dislocations, causing strain aging and temporary embrittlement.
③ In high chromium and high chromium-nickel steels, controlled nitrogen additions can enhance both strength and impact toughness without significant loss of plasticity. This is achieved through the formation of fine, stable chromium nitrides and the austenite-stabilizing effect of nitrogen.
④ Nitrogen improves the creep strength and high-temperature rupture strength of steel by forming thermally stable nitrides and solid solution strengthening of the matrix. This effect is particularly beneficial in austenitic stainless steels and heat-resistant alloys operating at elevated temperatures.
① The presence of nitrogen significantly impacts the corrosion resistance of stainless steel. Contrary to the original statement, nitrogen generally improves the pitting and crevice corrosion resistance, particularly in austenitic and duplex stainless steels. This improvement is attributed to nitrogen’s ability to stabilize the passive film and increase the pitting potential.
② When the nitrogen content exceeds approximately 0.2% by mass in austenitic stainless steels, the steel’s resistance to high-temperature oxidation may decline. However, the exact threshold depends on the specific alloy composition and intended application. In some cases, controlled additions of nitrogen up to 0.5% can enhance oxidation resistance at moderate temperatures.
③ Nitrogen-containing steel exhibits a higher work hardening rate during cold deformation. This phenomenon, known as the nitrogen strengthening effect, results from the interaction between nitrogen atoms and dislocations, leading to enhanced strength and hardness. This property is particularly beneficial in applications requiring high strength and wear resistance.
④ Nitrogen effectively reduces the tendency for grain growth in high chromium ferritic stainless steels, thereby improving their welding characteristics. It acts as an austenite stabilizer, promoting the formation of fine-grained structures during welding thermal cycles. This grain refinement enhances both the mechanical properties and corrosion resistance of the weld zone and heat-affected area.
Additionally, nitrogen improves the yield strength and ultimate tensile strength of stainless steels without significantly reducing ductility. It also enhances the steel’s resistance to stress corrosion cracking in chloride environments, making nitrogen-alloyed stainless steels particularly suitable for marine and chemical processing applications.
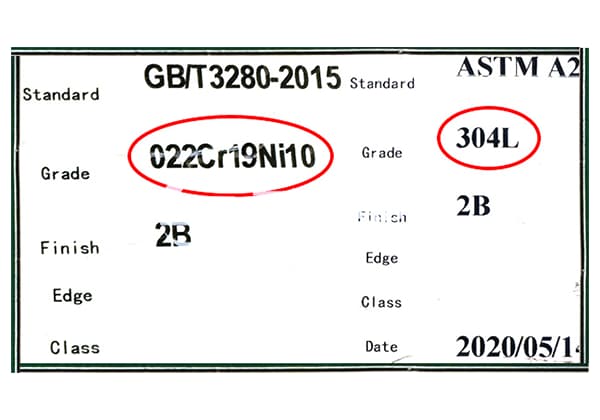
① Nitrogen serves as a crucial alloying element in steel, with its content typically ranging from trace amounts up to 0.3% (by mass) in most applications, and reaching a maximum of 0.6% in specialized high-nitrogen steels. This precise control of nitrogen content allows for tailored mechanical properties and microstructural characteristics.
② Nitrogen alloying finds extensive application in various steel types, including:
The addition of nitrogen offers unique benefits such as solid solution strengthening, grain refinement, and enhanced pitting corrosion resistance, making it an invaluable alloying element in modern steel metallurgy.