Have you ever wondered how steel transforms from a dull, rusty surface to a shiny, pristine finish? Steel pickling is the key process behind this remarkable metamorphosis. In this article, a seasoned mechanical engineer unveils the secrets of steel pickling, offering a fascinating glimpse into the world of metal surface treatment. Discover the science behind the process and its crucial role in various industries.

Recent regulatory actions by environmental protection agencies have intensified scrutiny on pickling operations within the metalworking industry. Facilities and processing plants failing to meet stringent environmental standards have faced closure orders or mandated rectification measures. This heightened oversight has sparked widespread concern among industry stakeholders regarding pickling processes and their environmental impact.
The pickling process, essential for removing surface oxides and impurities from metal substrates, traditionally involves the use of strong acids and hazardous chemicals. These substances, if not properly managed, can pose significant environmental and health risks. Key areas of concern include:
In response to these challenges, the industry is witnessing a shift towards more sustainable pickling technologies and practices. Innovations in this field include:

Pickling is a critical surface preparation process in metal manufacturing where iron oxide scales (rust and mill scale) are chemically removed from the metal surface using acid solutions. This process involves immersing the metal in a carefully controlled bath of acid at specific concentrations, temperatures, and exposure times. The acid reacts with the oxide layer, dissolving it and leaving a clean, bare metal surface.
Commonly used acids for pickling include hydrochloric acid (HCl), sulfuric acid (H2SO4), and phosphoric acid (H3PO4), each selected based on the metal type and desired surface finish. The process parameters—acid concentration, bath temperature, and immersion time—are precisely regulated to ensure effective scale removal while minimizing base metal loss and preventing over-pickling, which can lead to hydrogen embrittlement in some alloys.
Pickling is essential for preparing metal surfaces for subsequent processes such as plating, painting, or further fabrication, as it enhances coating adhesion and improves overall product quality and corrosion resistance.
Pickling is a critical surface treatment process in metal manufacturing, primarily used to remove oxides, scale, and impurities from metal surfaces. The classification of pickling methods can be categorized based on several factors:
1. By Acid Type:
• Sulfuric Acid (H2SO4) Pickling
• Hydrochloric Acid (HCl) Pickling
• Nitric Acid (HNO3) Pickling
• Hydrofluoric Acid (HF) Pickling
The selection of acid depends on the metal being treated. For example:
2. By Workpiece Geometry:
• Wire Pickling
• Forging Pickling
• Sheet/Plate Pickling
• Strip Steel Pickling
• Tube/Pipe Pickling
Each geometry presents unique challenges in acid circulation, immersion times, and handling methods.
3. By Equipment Configuration:
• Batch Tank Pickling
• Semi-Continuous Pickling
• Continuous Pickling Lines
• Spray Pickling
• Tower (Vertical) Pickling
The choice of equipment depends on production volume, material dimensions, and desired throughput. Continuous lines are often used for high-volume production of sheet and strip, while batch processes may be more suitable for varied or smaller production runs.
4. By Process Technology:
• Conventional Immersion Pickling
• Electrolytic Pickling
• Neutral Electrolytic Pickling (NEP)
• Turbulent Pickling
• High-Temperature Acid Regeneration (HTAR) Pickling
Advanced pickling technologies aim to improve efficiency, reduce environmental impact, and enhance surface quality.
The selection of the appropriate pickling method involves considering factors such as material composition, surface condition, production requirements, environmental regulations, and cost-effectiveness. Modern pickling operations often integrate automated control systems, acid recovery units, and fume scrubbers to optimize the process and minimize environmental impact.
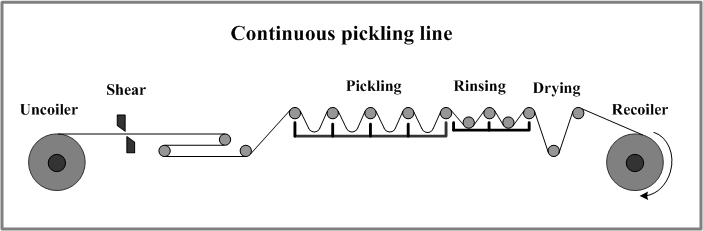
The removal of iron oxide scale can be accomplished through three primary methods: mechanical, chemical, and electrochemical. Each method offers distinct advantages and is suitable for different applications based on the scale characteristics, substrate material, and desired surface finish.
Mechanical Methods:
Chemical Methods:
Chemical descaling utilizes reactive substances to dissolve or loosen the iron oxide scale. Common chemical agents include:
The choice of chemical agent depends on the scale composition, base metal, and environmental considerations. Inhibitors are often added to protect the underlying metal from excessive attack.
Electrochemical Methods:
Electrochemical descaling, also known as electrolytic pickling, combines chemical and electrical processes:
This method offers faster descaling rates and can be more effective for tightly adhered scale layers compared to chemical pickling alone.
Each removal method has its strengths and limitations. The selection depends on factors such as scale thickness, substrate material properties, production volume, environmental regulations, and desired surface finish. Often, a combination of methods may be employed for optimal results in industrial applications.
At present, there are three main methods for pickling steel in the world:
Single-piece steel pickling is the most primitive and simple pickling method.
Its characteristic is that it does not need to open the coil.
The entire coil of wire or loose coiled steel coil is pickled and then hoisted in the pool for washing.
Due to the poor quality of pickling, the low efficiency of production, especially the environmental pollution, it is being vigorously eliminated.
Continuous pickling is a kind of high-yield, high-quality and fast-developing pickling method.
It is equipped with a welding machine and a looper device on the unit, so that the process section does not stop when the coil is changed to ensure the continuous operation of the unit.
But the unit is long, the equipment is complicated, and the investment is high.
There are two main types of continuous pickling lines, which are continuous horizontal pickling units and continuous tower pickling units.
Semi-continuous pickling is relative to single-piece and continuous pickling.
Since it does not need to set up a welding machine (or a simple welding machine such as a sewing machine) and a large-capacity looper, it is necessary to stop the machine when changing the coil.
This method is most suitable for the production of 200,000 to 900,000 t/a.
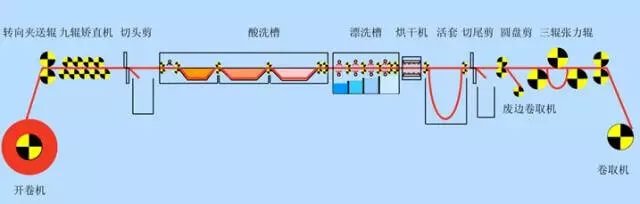
Fig.1 Production line layout diagram of push-pull pickling unit
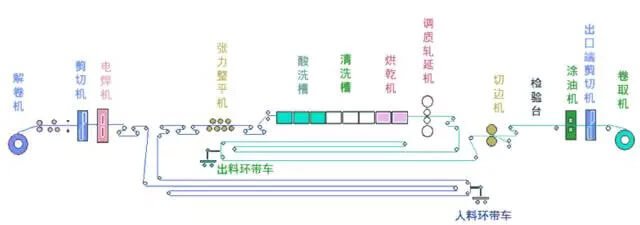
Fig.2 Production line layout diagram of full continuous pickling unit
As people’s living standards improve, the demand for a cleaner environment is increasing. To meet this need, new technologies and production lines with high efficiency, no pollution, and good quality for removing iron oxides are constantly emerging.
On July 20, 2013, with the roll-free pickling coil being rolled off the production line of Taiyuan Iron and Steel Group’s hot rolling mill, China’s first steel coil surface pickling-free treatment line was officially put into production.
In the past, the surface treatment of steel coils had to be completed by pickling and oiling, which had high production costs, caused environmental pollution, and resulted in waste disposal problems.
The newly-developed pickling-free treatment line uses the new EPS treatment technology, which eliminates the pickling process of steel coils, eliminates the generation of waste, recycles all the media, and makes the surface of the steel coils cleaner and more corrosion-resistant.
The EPS patent technology is developed based on SCS technology. The basic principle is to use a special device for EPS processing in a closed space. The upper and lower surfaces of the steel plate are sprayed with the EPS working medium, a mixture made of steel grit and water, and the oxide or scale on the surface of the steel plate is removed under a certain spraying force without any oxide remaining.
Therefore, the surface of the steel sheet can be made smooth and clean, which is called a “green” surface.
Pickling is the process of chemically removing the scale on the metal surface, so it is also called chemical pickling. The scales (Fe203, Fe304, Fe0) formed on the surface of the strip are all alkaline oxides that are insoluble in water. When the metal is immersed in an acid solution or sprayed with an acid solution on the surface, these basic oxides undergo a series of chemical changes with the acids.
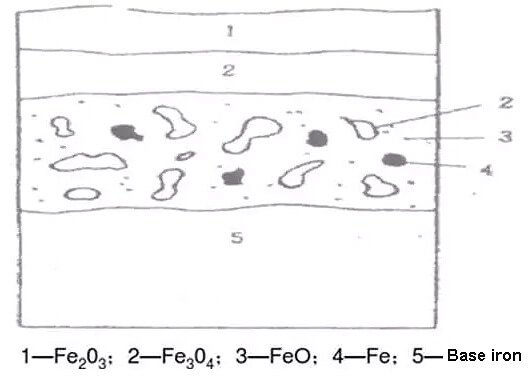
Fig.3 Structure of iron oxide scale
The scale on the surface of carbon structural steel or low-alloy steel is loose, porous, and cracked. In addition, the scale is repeatedly bent, straightened, and conveyed along with the strip on the pickling unit, which further increases and expands the pore cracks.
Therefore, while the acid solution chemically reacts with the iron oxide scale, it also reacts with the base iron of the steel through cracks and pores. That is to say, at the beginning of pickling, there are three kinds of chemical reactions between the iron oxide scale, metal iron, and acid solution.
Hydrochloric acid (HCl) has emerged as the predominant pickling medium in modern metal processing, surpassing sulfuric acid (H2SO4) in industrial applications due to its superior performance characteristics.
HCl pickling offers distinct advantages:
These factors have led to the widespread adoption of HCl pickling in various metal processing industries, including steel manufacturing, automotive, and aerospace sectors. However, the choice between HCl and H2SO4 pickling may still depend on specific application requirements, material composition, and local environmental regulations.
This ambient temperature rust remover quickly cleans rust and oxidation from iron surfaces and also has degreasing properties. Its composition and operating conditions are as follows:
Note: Table salt controls the corrosive action of H2SO4 on carbon steel, chromium steel, and chromium nickel steel, and also acts as a dust inhibitor.
To prevent acid mist, add a 10% mist suppressant.
This rust removal solution works quickly and, at room temperature, rust removal takes no longer than 10 minutes. Thanks to the dust and ash inhibitor, the metal surface is clean and displays a grey-white surface after pickling.
The Refinery, dealing with 200m, Φ200mm~300mm steel pipes lined with rubber, first carried out internal pipe rust removal using this rust remover, which proved to be very effective. All the rubber linings passed inspection.
Before applying a corrosion-resistant coating to a carbon steel water cooler, it needs to be pickled to remove rust. The washing solution composition is as follows (mass fraction):
Pickling Process: Room temperature pickling for 30~60 minutes, then rinse with water until neutral. Finally, carry out phosphating or passivation. This formula is widely used domestically for rust removal in water-cooled heat exchangers, with good coating results.
Rust Removal Process: At a temperature of 30~50°C, remove rust for 1 hour, then rinse with water until the pH value is 7. Finally, using a 10% solution of sodium nitrite at a temperature of 30~40°C, passivate for 30 minutes to prevent rusting.
Rust Removal Process: At a temperature of 40~50°C, remove rust for 15~16 minutes, then rinse with water. This formula is mainly suitable for rust removal from high-alloy steels.
Pickling Process: Heat the rust removal solution to 85~95°C and pickle for 2~3 minutes to remove rust. This formula is mainly suitable for the removal of light rust from precision parts, bearings, and the like.
Rust Removal Solution Operating Conditions: Rust removal temperature 80~90°C, rust removal time, 10~20 minutes.
With the development of industry, “two-in-one” oil removal and rust removal, “three-in-one” oil removal, rust removal, phosphating or passivation multi-functional rust removers have emerged.
Rust Removal Process: Room temperature pickling rust removal for 2~10 minutes, then rinse with water until neutral, and finally carry out rust prevention treatment.
Its formula is shown in the following table:
Table 1 Rust Removal Solution Formula
| Raw material name | Content 1% | Forgive Name | Content/% |
| Disodium hydrogen phosphate Sodium nitrite Sodium bicarbonate | 3.5 6.2 1 | Glycerol Water | 1.6 87.7 |
This rust prevention solution has strong rust prevention capability, but the rust prevention time is relatively short, so it is suitable for rust prevention between processes.
The “three-in-one” degreasing, rust removal, and passivation (or phosphating) solution is suitable for metal equipment treatment before painting, thereby achieving the purpose of degreasing, rust removal, and passivation (or phosphating). However, it is not suitable when there is a lot of scale and heavy rust spots.
“Three-in-one” Degreasing, Rust Removal, and Passivation Composition:
The working conditions are rust removal temperature 85°C, rust removal time 2~2 minutes.
“Three-in-one” Degreasing, Rust Removal, and Phosphating Solution Composition:
The working conditions are a temperature of 85°C and a time of 2~3 minutes.
The composition of the “four-in-one” degreasing, rust removal, phosphating, and passivation solution is shown in the following table.
| “Four-in-one” Formula/(g/L) | Rust Removal Temperature/°C | Rust Removal Time/min | ||||
| Material Name | 1# | 2# | 1# | 2# | 1# | 2# |
| Phosphoric Acid (80% Content) | 110~180 | 110 | 50~60 | 55~65 | 25 | 5~15 |
| Zinc Oxide | 30~50 | 25 | ||||
| Zinc Nitrate | 150~170 | 150 | ||||
| Magnesium Chloride | 15~30 | 3 | ||||
| Potassium Tartrate | 0.2~0.4 | 5 | ||||
| Ammonium Molybdate | 0.8~1.2 | 1 | ||||
| Sodium Dodecyl Sulfate | 20~40 | 30 | ||||
| Manganous Phosphate | / | 10 | ||||
| Potassium Dichromate | / | 0.2~0.3 | ||||
| Water | Residual | Residual | ||||
Take formula 2# as an example, pour a certain amount of zinc oxide into a container and make it into a paste with distilled water. While stirring, add phosphoric acid until it dissolves into transparent zinc dihydrogen phosphate. Dilute with distilled water to 2/3 of the total volume, then add calculated amounts of zinc nitrate, magnesium chloride, phosphoric acid, tartaric acid, and potassium dichromate. After stirring until dissolved, add the solution of ammonium molybdate that has been dissolved in a small container in two batches, and stir evenly. Finally, add 601 cleaning agent and dilute to the total volume.
The 601 cleaning agent is an anionic surfactant, which has good permeability and wettability, is easily soluble in water, acid-resistant, heat-resistant, and does not react with metal ions, so it is very stable in the solvent and is used for oil removal. The phosphating process of the “four-in-one” is the same as the general phosphating principle. Oil removal and rust removal occur at the same time, and phosphoric acid has a soaking effect on iron, forming a dense phosphating film on the surface of the steel. Ammonium molybdate and potassium dichromate act as passivators.
The free acidity of the “four-in-one” treatment solution is 17~25 points, the total acidity is 170~220 points, and the ratio of free acidity to total acidity is (1:7)~(1:10).
Use 0.1mol/L NaOH standard solution to titrate 10mL of phosphating solution. When phenolphthalein is used as an indicator, the milliliters of NaOH consumed is the “point” number of total acidity. When methyl orange is used as an indicator, the milliliters of NaOH consumed is the “point” number of free acidity.
Steel parts with gold and rainbow colors can be processed directly. If the oil pollution is serious, OP emulsifier can be added to enhance the decontamination ability. Steel parts with blue oxide scales cannot be treated with this method because the oxide scale is too thick. The amount of scale that this method can dissolve is 7~10g/m².
On the surface of steel parts treated with “four-in-one”, a thin film of insoluble phosphates of metals such as zinc, iron, and magnesium is formed. This film has protective properties and can adsorb paint, which improves the adhesion of the paint film and enhances protective capabilities.
Next, I will introduce two more “four-in-one” degreasing, rust removal, phosphating, and passivation solution formulas suitable for removing oxide scales.
The process of the first formula is more complex than other formulas. It is divided into two steps. The first step is to remove oil and rust (oxide scale) (see Table 1 for the formula); the second step is phosphating and passivation treatment (see Table 2 for the formula). It should be noted that after the oil and rust are removed, you should first rinse with water. After the water is neutral, phosphating and passivation treatments can be carried out.
Table 1 Formula for removing oil and rust (oxide scale)
| Oil and Rust Removal Formula | Working Conditions for Oil and Rust Removal Solution | ||
| Ingredient | Content | Rust Removal Temperature /°C | Rust Removal Time /min |
| Industrial Sulfuric Acid (Relative Density 1.84) | 60~65mL/L | 75~85 | 5~20 |
| Thiourea | 5~7g/L | ||
| Sodium Dodecylbenzenesulfonate | 20~50mL/L | ||
| Water | Residual | ||
Note: The amount of sulfuric acid can be increased if the oxide scale is thick.
Table 2 Phosphating and passivation solution formula
| Phosphating and Passivation Formulation | Phosphating and Passivation Working Conditions | ||
| Ingredients | Concentration/(g/L) | Phosphating Temperature /°C | Phosphating Time /min |
| Phosphoric Acid | 58 | 65~75 | |
| Zinc Oxide | 15 | ||
| Zinc Nitrate | 200 | ||
| Dihydrogen Chromium Phosphate (calculated as Potassium Dichromate) | 0.3~0.4 | ||
| Titanium Sulfate Oxide (not necessary for welding) | 0.1~0.3 | ||
| Tartaric Acid | 5 | ||
| Sodium Dodecyl Sulfate | 15mL | ||
| OP Emulsifier | 15mL | ||
| Water | Residual | ||
Note: The ratio of free acidity to total acidity: (1:12) ~ (1:18)
Phosphating process parameters: Use a 1:1 dilution of the phosphating solution with water, treatment time is 15~30 minutes, treatment temperature is 10~60°C, total acidity is 200~250 points.
Drying time: natural drying for 24 hours, or drying at 120°C for 30~60 minutes. The appearance of the phosphating film is dark gray, the film is densely crystallized, continuous and uniform; the phosphating film thickness is 5~8μm; the adhesion is grade I; the impact resistance is 500N·cm; the copper sulfate drop test >150S; the sodium chloride solution soaking test >8h; indoor rust prevention (T20°C RH86%) >60 days.
Acid pickling paste can be used to remove rust. The recommended application thickness is 1~2mm, with an amount of 2~3kg/m², and the total rust removal time is 60 minutes. After rust removal, rinse with water.
Finally, wipe dry with a mixture of acetic acid and ammonia solution to aid in rust prevention. The formula for the acid pickling paste is as follows (please provide the table):
Table 3 Rust Prevention Acid Pickling Paste Formula
| Per 300g quantity /g | Per 300g quantity /g | ||
| Industrial Hydrochloric Acid (30% concentration) | 4.3 | 1.2 | 1% |
| Phosphoric Acid (Relative density 17) | 18.6 | 0.2 | / |
| Industrial Sulfuric Acid (Relative density 1.84) | 40.3 | 5.5 | 5.33% |
| Hexamethylenetetramine | 0.8 | 0.1 | 0.10% |
| Bentonite (120#) or Diatomaceous Earth, Yellow Ochre | 200 | 200 | 7.60% |
| Oxalic Acid | / | / | 0.07% |
| Asbestos Wool | / | / | 6.50% |
| Water | 36 | 93 | Residual |