Have you ever marveled at the mesmerizing colors of heated steel? The vibrant reds, oranges, and yellows tell a fascinating story about temperature. In this article, we’ll explore the science behind steel’s color changes during heating, drawing from the expertise of experienced metallurgists. Discover how understanding these color-temperature relationships can help you master heat treatment processes and produce high-quality steel components. Get ready to see steel in a whole new light!
The color temperature of steel is closely related to the heating process. At room temperature, steel does not emit light. However, when heated to a certain temperature, it begins to glow, initially emitting a red light. As the temperature rises further, the color of the steel gradually changes from red to orange, and then to yellow.
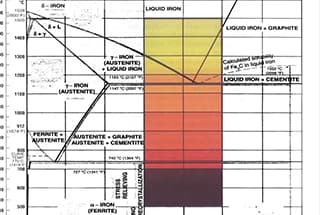
This process aligns with the concept of black-body radiation, where color temperature is defined based on black-body radiation, with orange-yellow having a lower color temperature and blue having a higher one.
Specifically for steel, when its color temperature reaches 3200K, the color of the light is relatively close to red, which is the color of iron when heated to over a thousand degrees.
If the heating continues, the glow will become brighter and the color will get closer to white.
This indicates that by controlling the heating process, a color change from red to near white can be achieved.
This is not an accurate method and it may vary depending on the type of steel being used. These colors are only applicable for certain types of steels (probably carbon steel). The color of the flame can be different for different types of metals at the same temperature.
In 1893, Wien studied the relationship between the maximum wavelength λmax and temperature T, which is λmaxT=2898μm•K.
Therefore, the temperature can be judged based on the color of the flame (i.e., the wavelength of light).
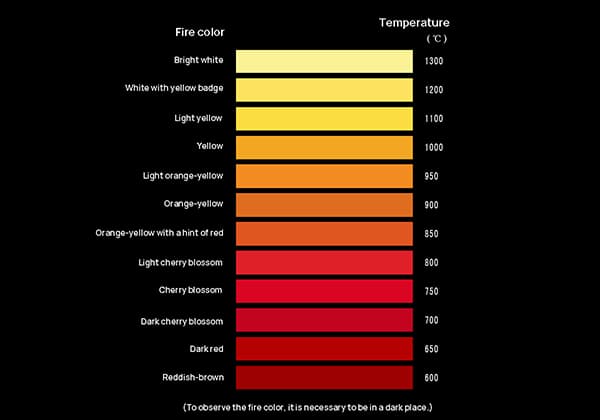
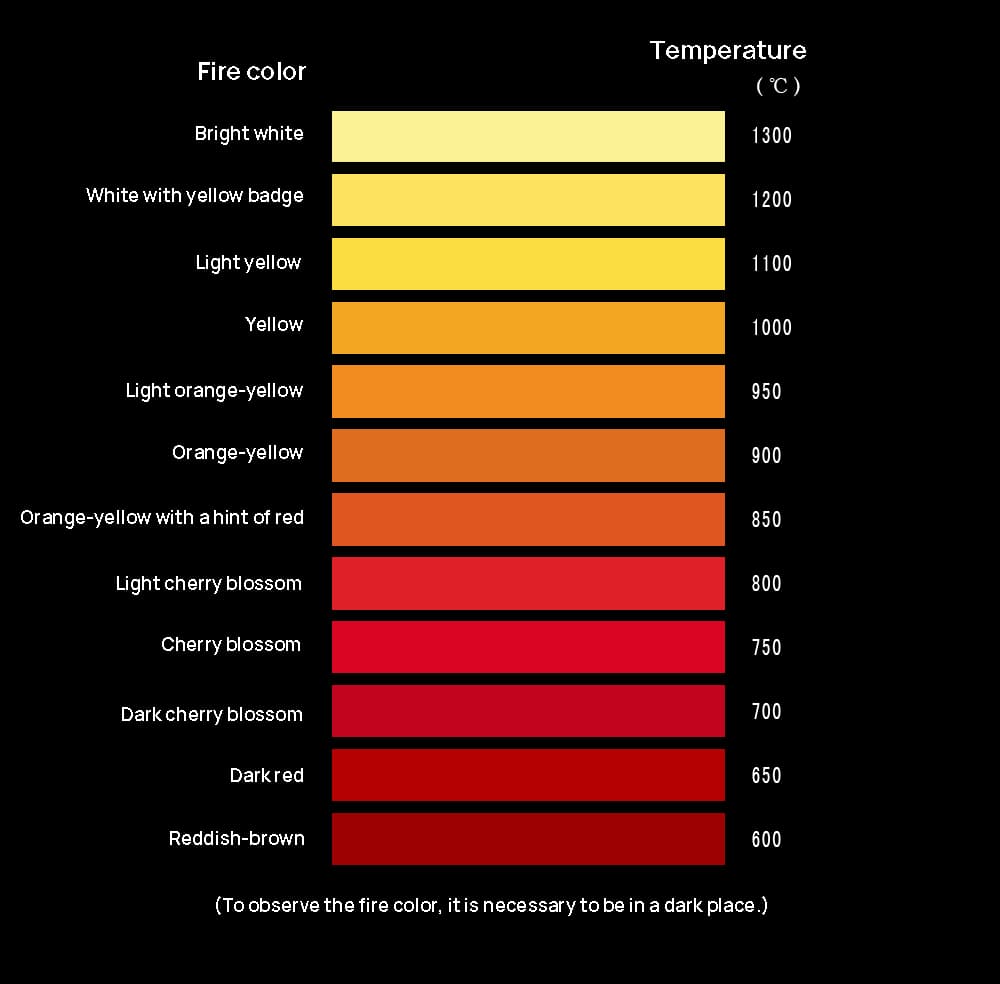
Empirical observation shows that dark red indicates 600°C, red indicates 900°C, orange-yellow indicates 1100°C, yellow indicates 1300°C, light yellow indicates 1400°C, yellow-white indicates 1500°C, and bright white (with a hint of yellow) indicates 1600°C.
There is a type of temperature-sensitive paper developed by Nichiyu Giken Kogyo Co., Ltd. that can be placed on the heated metal to show its temperature changes through different colors.
By observing the color changes of the paper on different parts of the metal, one can determine their respective temperatures and record them accordingly to make a color chart for further use.
The Relationship Between the Color of Steel Heating and Temperature
| Fire color | Temperature ℃ |
| Dark brown | 520——580 |
| Dark red | 580——650 |
| Dark cherry | 650——750 |
| Cherry blossom | 750——780 |
| Light cherry blossom | 780——800 |
| Light red | 800——830 |
| Orange-yellow with a hint of red | 830——850 |
| Light withered | 880——1050 |
| Yellow | 1050——1150 |
| Light yellow | 1150——1250 |
| Yellow-white | 1250——1300 |
| Bright white | 1300——1350 |
The relationship between the tempering color and temperature of carbon steel.
| Tempered color | Temperature ℃ |
| Light yellow | 200 |
| Yellow-white | 220 |
| Golden yellow | 240 |
| Yellow-purple | 260 |
| Dark purple | 280 |
| Blue | 300 |
| Dark blue | 320 |
| Blue-gray | 340 |
| Blue-gray light white | 370 |
| Black-red | 400 |
| Black | 460 |
| Dark black | 500 |
This seems to require a lot of experience, as the temperatures can be different during the day and night. The thermometer is not always easy to use and may not be very accurate.
There can also be differences between the temperature of the flame and the temperature of the object being measured.
The color of glowing steel is a reliable indicator of its temperature, used extensively in metalworking to ensure the correct heat treatment processes. This chapter outlines the specific temperature ranges associated with different shades of red, providing practical examples and applications to help you understand their significance.
At this initial heating stage, steel emits a black red glow. This temperature range is suitable for preliminary heating and softening of the steel, preparing it for further processing. Although it is not yet ideal for extensive forging, it can be used for basic shaping tasks. For example, black red heat is often used to soften steel before cutting or to relieve internal stresses in the material.
As the temperature increases, steel reaches a very dark red glow. This range is often used for initial forging processes where the steel begins to be more malleable. It is suitable for rough shaping and preliminary forging operations. For instance, blacksmiths may use this temperature to start forming the basic outline of a tool or blade.
When steel reaches a dark red glow, it becomes ideal for more extensive forging. This temperature range allows for significant deformation without compromising the steel’s integrity. It is commonly used for general blacksmithing tasks such as bending and forming. At this stage, steel can be shaped into more complex forms, such as hooks, brackets, or intricate decorative elements.
A cherry red glow indicates that the steel is at an optimal temperature for processes like drawing-down and upsetting. This range provides the right balance of heat for achieving desired shapes and sizes while maintaining the steel’s structural properties. It is also a critical temperature for certain heat treatment processes, ensuring the steel attains specific mechanical characteristics. For example, cherry red heat is essential for achieving the correct hardness and toughness in cutting tools.
At a light cherry red glow, steel is suitable for detailed forging and precise bending. This temperature range is particularly useful for tasks requiring high malleability and fine control over the material’s shape. It is also approaching the temperature range needed for welding operations. For instance, light cherry red heat is often used in the final stages of forging to refine the shape and dimensions of a piece.
Beyond the red range, steel continues to change color at higher temperatures:
These additional color indications are used for processes requiring even higher temperatures, such as certain types of welding and advanced forging techniques.
Understanding these temperature ranges and their corresponding colors is crucial for metalworkers to ensure proper heating and treatment of steel. This knowledge allows for precise control over the material’s properties, leading to high-quality and reliable outcomes in various metalworking applications.
There are several methods to accurately measure the color temperature of steel:
1. Color Temperature Meter:
A color temperature meter is a tool specifically used for measuring the color temperature of a light source. Its usage is similar to a light meter, mainly by placing the measuring probe on the object to be measured. This method is suitable for directly measuring the intensity of all wavelength light emitted by the light source, thus obtaining the color temperature value.
2. Spectral Analysis:
Spectral analysis measures the color temperature by directly measuring the intensity of all wavelength light emitted by the light source. This method can provide more detailed spectral information, helping to accurately assess the color temperature of steel.
3. Colorimeter:
A colorimeter is another tool specifically used for measuring the color temperature of a light source, including filter-type and crystal-type. The filter-type colorimeter measures the color temperature by filtering specific wavelength light, while the crystal-type colorimeter determines the color temperature by measuring the crystal’s response to different wavelength light.
Accurate measurement of the color temperature of steel can be achieved by using a color temperature meter, spectral analysis, or a colorimeter. The choice of method depends on the specific measurement requirements and available resources. For example, if you need to get results quickly and the accuracy requirement is not very high, you can choose a color temperature meter; if more detailed spectral information is needed for in-depth analysis, spectral analysis may be more suitable; and if you have very high requirements for the accuracy of the measurement results, consider using a colorimeter for precise measurement.
The detailed changes in the luminescent properties of steel at different temperatures can be understood from several aspects. Firstly, when the metal reaches a certain temperature, the movement of its internal particles becomes violent, which may cause photons to reach the minimum frequency of visible light, thus producing red luminescence. This indicates that at lower temperatures, steel may not glow or the light intensity may be weak, as the change in electron energy levels is not enough to produce visible light.
As the temperature rises, the luminescence intensity of the phosphor will decrease due to the thermal quenching phenomenon. This phenomenon is mainly due to the increase in temperature causing the matrix lattice vibration to intensify, enhancing the electro-acoustic interaction and the probability of non-radiative transition, thus reducing the light intensity. Although phosphors are mentioned here, this principle also applies to metal materials such as steel, and a decrease in luminescence intensity may be observed at high temperatures.
In addition, from the perspective of luminescence studies, changes in temperature have a significant impact on refrigeration efficiency, and this impact has a cubic relationship with temperature. This means that as the temperature decreases, the difference between the optimal excitation light frequency and the center frequency of the non-uniform line shape will increase, reaching a maximum at lower temperatures. This indicates that under low-temperature conditions, the luminescent properties of steel may vary due to excitation at specific frequencies, especially at low temperatures, where it may be easier to observe the luminescence at specific wavelengths.
The luminescent properties of steel will change at different temperatures as follows: at lower temperatures, due to the insufficient change in electron energy levels to produce visible light, steel may not glow or the light intensity may be weak; as the temperature rises, due to the intensification of lattice vibrations and the increase in electro-acoustic interaction, the luminescence intensity of steel may decrease; and under low-temperature conditions, excitation at specific frequencies may cause steel to exhibit different luminescent properties, especially at low temperatures, where it may be easier to observe the luminescence at specific wavelengths.
The relationship between color temperature and blackbody radiation theory during the heating process of steel can be explained from the following aspects:
Definition of color temperature: Color temperature is a scale that measures the color of a light source, and its unit is Kelvin. It is determined by comparing the color of the light source with a theoretical thermally radiating blackbody. The Kelvin temperature at which the thermal radiating blackbody matches the color of the light source is the color temperature of that source.
Blackbody radiation theory: A blackbody is an idealized object that can absorb all radiation energy falling on it without loss and can radiate energy in the form of electromagnetic waves. Planck’s law describes the theoretical distribution of wavelengths in blackbody radiation, that is, as the temperature changes, the color of light will also change.
Color temperature changes during the heating process of steel: During the heating process of iron, the black iron gradually turns red. This is because as the temperature rises, the blackbody can emit all visible light waves in the spectrum, leading to color change. This process is an example of blackbody theory, illustrating the relationship between color temperature and temperature changes during the heating process of an object.
In practical applications, the selection of suitable steel materials based on color temperature requires the consideration of multiple factors. For instance, in the design of streetlights, choosing steel materials with an appropriate color temperature can enhance the effectiveness of road illumination, making the roads safer and easier to navigate. If the steel used in the streetlights has a high color temperature (cool tones), it might provide a clearer field of vision, but at the same time, it could reduce the warmth of the nighttime environment. On the contrary, steel with a low color temperature (warm tones) might increase the warmth of the environment, but it could affect visibility.
Furthermore, the choice of thermoforming temperature is crucial to ensure the quality of the formed parts. Different steel materials have different temperature-mechanical property curves, meaning that the physical state of the steel changes during the heating process, affecting its final shape and quality. Therefore, when choosing steel materials, it’s also necessary to consider the heat treatment requirements during its processing to ensure the material can meet specific application demands without sacrificing performance.
When selecting suitable steel materials based on color temperature, it’s important to consider the visual effects of the material, its physical and chemical properties, and the heat treatment requirements during its processing. By carefully evaluating these factors, one can choose the steel material that best suits the specific application requirements.
The impact of steel color temperature on product performance is mainly reflected in the following aspects:
1. Heat treatment process of mold steel:
The color of mold steel does not change at low temperatures, but when heated to about 600℃ and above, a slight dark red color appears. As the temperature rises, the color of the mold steel gradually changes. This shows that the color temperature change of the steel is related to the performance change during the heat treatment process, and the color change indirectly reflects the changes in the internal structure and performance of the material.
2. Changes in the strength and plasticity of steel:
An increase in temperature leads to a reduction in the strength of steel, and an increase in deformation. Especially near 250℃, the tensile strength of steel increases, but plasticity and toughness decrease, while a blue brittleness phenomenon occurs, that is, the oxide film turns blue. This phenomenon shows that the color temperature change of steel at a specific temperature (such as the color change of the oxide film) is closely related to its mechanical performance changes, especially the changes in tensile strength, plasticity, and toughness.
Below are answers to some frequently asked questions:
Steel first starts to glow red at approximately 525°C (977°F). This temperature marks the onset of visible red heat, which can vary slightly depending on lighting conditions and specific steel composition. As the temperature increases, the red glow becomes more pronounced, transitioning from a dull red to a brighter cherry red up to around 704°C (1,299°F).
The color of glowing steel changes with temperature due to incandescence and the formation of oxide layers. When steel is heated, its atoms become energized and emit photons, causing the metal to glow. The specific color of the glow is directly related to the temperature of the steel.
At lower temperatures, around 525°C (977°F), steel begins to show a faint red glow. As the temperature increases to between 700°C and 870°C (1,300°F to 1,598°F), the glow progresses from dark red to cherry red. At around 981°C (1,798°F), the color becomes light cherry red.
As the temperature continues to rise, the color changes to orange. Deep orange is observed at approximately 1,100°C (2,010°F), and clear orange appears up to 1,200°C (2,190°F). When the temperature reaches between 1,093°C and 1,258°C (1,999°F to 2,296°F), the steel glows yellow. At about 1,314°C (2,397°F), it shifts to yellow-white, and at temperatures above 1,315°C (2,397°F), it becomes white. Beyond 1,400°C (2,550°F), the steel can glow white bright to dazzling white.
At temperatures below the incandescence range, the color changes are due to the formation of oxide layers on the steel’s surface. This oxidation process results in different colors depending on the thickness of the oxide layer. For example, during tempering, reheating the steel to lower temperatures can produce colors such as blues and yellows, which indicate the degree of hardness achieved.
In summary, the color of glowing steel changes with temperature due to incandescence at higher temperatures and oxide layer formation at lower temperatures. These color changes are useful for determining the temperature and structural state of the steel during processes like heat-treating and tempering.
Knowing the temperature at which steel glows red is crucial for several reasons, especially in blacksmithing, heat treating, and metalworking.
The color of glowing steel serves as a visual indicator of its temperature. Red is one of the first visible colors that appear as the metal is heated, corresponding to specific temperature ranges. For instance, steel starts to glow red at temperatures ranging from about 799°F (426°C) to 1,100°F (593°C), depending on the shade of red.
In blacksmithing and forging, understanding these temperature ranges is essential for achieving the desired properties of the metal. Different shades of red indicate optimal temperatures for various processes. For example, “cherry red” (approximately 1,498°F to 1,598°F or 815°C to 870°C) is often used for forging and shaping steel, while darker shades of red are associated with lower temperatures used in different stages of heat treatment and tempering.
The temperature at which steel glows red is also linked to its structural and mechanical properties. Precise temperature control during heat treating processes, such as tempering, is necessary to achieve the desired hardness, toughness, and other characteristics of the steel. For example, tempering steel at specific temperatures can relieve internal stresses and modify its chemical properties.
Accurate temperature control is important for safety and efficiency. Recognizing color indicators helps blacksmiths and metalworkers ensure that the metal is heated to the optimal temperature, preventing overheating or underheating. This enhances the efficiency of the process and reduces the risk of undesirable outcomes such as brittleness, softening, or damage to the metal.
Historically, before the widespread use of thermometers, the color of glowing metal was the primary method for determining temperature. This practice, rooted in blacksmithing traditions, allowed craftsmen to heat metals to precise temperatures without modern measuring tools.
In summary, knowing the temperature at which steel glows red is vital for accurate heat treating and forging, ensuring the desired material properties, and maintaining safety and efficiency in metalworking.
Knowing the red glow temperature of steel and its associated color changes is crucial in metalworking due to several practical applications. In heat treatment, recognizing the color-temperature relationship allows metalworkers to precisely control processes like hardening, tempering, and annealing without relying on thermometers. Specific colors correspond to certain temperature ranges, which are essential for achieving the desired properties in steel. For instance, during tempering, colors like straw, yellow, and blue indicate optimal temperatures to enhance ductility and toughness while reducing brittleness.
In blacksmithing, being able to gauge the temperature by the color of the steel is vital for effective forging and shaping. Red heat (around 500-800°C or 932-1472°F) is typically used for forging, whereas higher temperatures are needed for more intense shaping. The color changes also indicate when the steel is ready for quenching, crucial for hardening.
For tool making, understanding the color-temperature relationship is key to producing tools with the correct properties. Different tools require specific tempering temperatures, indicated by colors such as light yellow for knives and razors (around 210°C or 410°F) and red-brown for taps and dies (around 260°C or 500°F). This knowledge helps maintain tool performance and longevity.
In industrial settings, observing color changes aids in troubleshooting and quality control. Discoloration can signal overheating or uneven heating, potentially leading to structural weaknesses. Monitoring these changes helps identify and address issues before they escalate. The color of the oxide layer can also reveal internal stresses or improper heat treatment, allowing for corrective measures.
Overall, knowing steel’s red glow temperature and related color changes is essential for precise heat treatment, effective blacksmithing, tool making, and ensuring the quality and performance of metal products.
The color that steel glows, including red, is primarily determined by its temperature rather than the specific type of steel. This phenomenon is based on black-body radiation, where the color changes predictably with temperature increases. Steel, regardless of its type—whether it is mild steel, carbon steel, or alloy steel—will glow red when it reaches a temperature of around 460°C (900°F). As the temperature continues to rise, the color will progress through shades of orange, yellow, and eventually white.
The consistency of the temperature-color relationship across different types of steel means that specific compositions do not significantly affect the glowing color at a given temperature. For instance, the “cherry red” color, which occurs at temperatures between 1500°F and 1800°F, is a common reference for various carbon steels.
However, there are some exceptions related to specific properties of the steel. For example, carbon steel with high sulfur content can exhibit a phenomenon known as red-short or hot-short, where the steel becomes brittle at red-hot temperatures due to the formation of iron sulfide. This brittleness does not alter the temperature at which the steel glows red but affects its mechanical properties at that temperature.
In summary, all types of steel will glow red at the same temperature range, dictated by black-body radiation principles, regardless of their specific compositions.