Which material reigns supreme in hardness: tungsten carbide or diamond? This blog explores the fascinating world of these two extraordinary substances. From their unique applications in jewelry and industry to their unparalleled physical properties, you’ll uncover the secrets behind their enduring appeal and discover which one truly stands the test of time.

Tungsten and diamond exhibit significant differences in hardness, durability, and appearance. Diamond, a pure carbon allotrope, is the hardest known material, ideal for jewelry and industrial cutting tools. Tungsten, however, known for its extreme durability and high melting point, is largely used in electrical applications and heavy-duty tools. It is less brilliant but more affordable than diamond.
Diamond, also referred to as “adamant”. It is a mineral composed of carbon elements and is an allotropic form of carbon.
Diamond is the hardest naturally occurring substance in nature. But when compared to carbides, which one has superior hardness? Let’s discuss which is harder, tungsten carbide or diamond.

Many people are aware that tungsten carbide is hard. Products processed using tungsten carbide greatly enhance their hardness, durability, and wear resistance.
Many people don’t have a strong concept of the hardness of different materials and only know that diamond is the hardest substance.
In fact, adamant is just another name for diamond, so tungsten carbide is certainly not as hard as adamant.
Although tungsten carbide is not as hard as diamond, its hardness is still quite exceptional.
Tungsten steel (hard alloy) has high hardness, wear resistance, good strength, and toughness, heat resistance, corrosion resistance, and a series of excellent properties, especially its high hardness and wear resistance, which remain virtually unchanged even at 500℃ and still maintain high hardness at 1000℃.
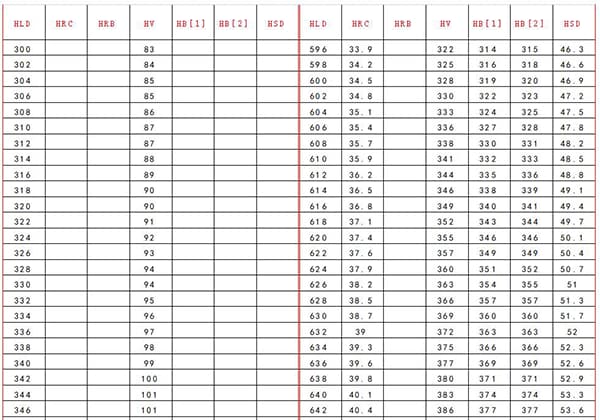
Tungsten carbide, with a Mohs hardness of about 9 to 9.5, is among the hardest substances. By comparison, the Mohs hardness of diamond is 10, with the hardness of diamond being the highest benchmark.
While tungsten carbide is not as hard as diamond, some of its other physical parameters are much better than diamond. For example, its stiffness can reach twice that of steel, with a Young’s modulus of about 530-700 GPa, which is also twice that of steel.
It is because of its high hardness and excellent other properties that tungsten carbide has found extensive applications in fields like aerospace, petroleum and natural gas, chemical engineering, fluid control, and heavy machinery.

Diamond also has a wide range of uses, such as in crafts, industrial cutting tools, and more. Graphite can form synthetic diamond under high temperature and high pressure.
Its industrial uses are also quite common, including geological and oil drilling diamonds, wire drawing diamond dies, abrasive diamonds, truing diamonds, glass knives with diamonds, hardness tester diamond indenter, craft diamonds, and so on.
Tungsten carbide is a product of powder metallurgy, created by sintering a compound of carbon and tungsten, which are both high in hardness and melting point, with binders such as cobalt (Co), molybdenum (Mo), and nickel (Ni).
Its hardness at room temperature can reach 78-82 HRC, and it can withstand high temperatures of 850-1000℃, with cutting speeds 4-10 times higher than high-speed steel.
However, its impact toughness and bending strength are significantly lower than those of high-speed steel, making it less likely to be used as a solid tool.
Pure tungsten carbide is not commonly used. It appears as a black hexagonal crystal with a metallic luster and a hardness close to that of diamond.
It conducts heat and electricity well, with a melting point of 2870℃ and a boiling point of 6000℃. It has a relative density of 15.63 at 18℃.
Tungsten carbide is insoluble in water, hydrochloric acid, and sulfuric acid, but dissolves easily in a mixture of nitric acid and hydrofluoric acid. When small amounts of metals such as titanium and cobalt are added to tungsten carbide, its brittleness decreases.
Tungsten carbide used for cutting steel often contains titanium carbide, tantalum carbide, or a mixture of both, to improve its explosion resistance. Tungsten carbide is chemically stable.
In tungsten carbide, the carbon atoms fill the gaps in the tungsten metal lattice without disrupting the metal’s original lattice, forming an interstitial compound.
Tungsten carbide is suitable for machining at high temperatures and can be used to make cutting tools, structural materials for furnaces, jet engines, gas turbines, nozzles, and more.

Diamond is unequivocally harder than tungsten, maintaining its position as the hardest known natural material.
Tungsten carbide, often referred to as simply “tungsten” in jewelry applications, ranks as one of the hardest metallic substances, second only to diamond in overall hardness. The exceptional hardness of tungsten carbide jewelry is a defining characteristic, surpassing 10K gold by a factor of 10, stainless steel by 5 times, and titanium by 4 times on the Mohs scale of mineral hardness.
Tungsten carbide, an alloy composed of tungsten and carbon atoms, exhibits remarkable hardness and stability. Its hardness approaches that of diamond, typically measuring between 8.5 and 9.5 on the Mohs scale, compared to diamond’s perfect 10. When expertly crafted, tungsten carbide jewelry possesses a distinctive, enduring luster that sets it apart from other metallic accessories.
Wearers of tungsten carbide jewelry often note that the material’s appearance improves with prolonged skin contact. Unlike many metals, tungsten carbide resists oxidation, maintaining its original appearance or even developing an enhanced sheen over time. This unique property, combined with its extreme hardness, renders tungsten carbide highly resistant to everyday wear and tear. The material’s durability is such that it can withstand contact with sharp objects like knives or exposure to strong acids without showing visible damage or degradation.
It’s important to note, however, that while tungsten carbide is exceptionally hard and scratch-resistant, it lacks the toughness of some softer metals. This means that under extreme stress, tungsten carbide can potentially crack or shatter rather than deform, a characteristic that should be considered in certain high-impact applications.
Diamond is unequivocally harder than tungsten steel, representing the pinnacle of natural material hardness.
Tungsten steel, also known as tungsten carbide or cemented carbide, is a composite material primarily consisting of tungsten carbide (WC) particles bonded with a cobalt matrix. This composition typically yields a hardness range of 89-95 HRA (Rockwell A scale) or approximately 9.0-9.5 on the Mohs scale. The exceptional hardness of tungsten steel stems from the covalent bonding in tungsten carbide crystals and the binding properties of the cobalt binder. This combination imparts high wear resistance, thermal stability, and compressive strength, making it ideal for cutting tools, wear parts, and high-performance components in various industries.
Despite its impressive hardness, tungsten steel exhibits some brittleness due to its ceramic-like nature. This characteristic limits its application in scenarios requiring impact resistance or ductility. The material’s composition can be fine-tuned by adjusting the carbide grain size, cobalt content, and addition of other carbides (e.g., titanium carbide, tantalum carbide) to optimize specific properties for different applications.
Diamond, in contrast, represents the zenith of material hardness. On the Mohs scale, diamond defines the maximum hardness of 10, while its absolute hardness on the Knoop scale ranges from 7000 to 10,000 KHN (Knoop Hardness Number), depending on the crystal orientation. This extraordinary hardness results from diamond’s unique crystal structure – a three-dimensional network of carbon atoms, each covalently bonded to four others in a tetrahedral arrangement. This configuration creates an extremely rigid and stable lattice, explaining diamond’s unparalleled hardness and other remarkable properties.
The extreme hardness of diamond translates into exceptional wear resistance and cutting ability, making it invaluable for industrial applications such as precision cutting tools, abrasives, and wear-resistant coatings. In addition to its hardness, diamond possesses other notable characteristics:
While natural diamonds form under high pressure and temperature conditions in the Earth’s mantle, synthetic diamonds can be produced through various methods, including High-Pressure High-Temperature (HPHT) synthesis and Chemical Vapor Deposition (CVD). These techniques have revolutionized the availability of industrial-grade diamonds and opened new avenues for diamond applications in cutting-edge technologies.
It’s worth noting that while diamond is the hardest natural material, it can be brittle under certain conditions due to its perfect cleavage planes. This property is exploited in diamond cutting and shaping processes.
In conclusion, while tungsten steel represents one of the hardest engineered materials available, diamond significantly surpasses it in terms of hardness, cementing its position as the hardest known natural substance.

Diamonds are extracted globally, with over 30 countries possessing diamond resources. The annual global production averages approximately 130 million carats. The top five diamond-producing countries are Russia, Botswana, Democratic Republic of Congo, Australia, and Canada, collectively accounting for about 75% of the world’s diamond production by volume.
Russia leads global production, with significant deposits in Siberia. Botswana, known for its high-quality diamonds, is the second-largest producer by value. The Democratic Republic of Congo, while third in volume, primarily yields industrial-grade diamonds. Australia, once the leading producer, has seen a decline but remains significant. Canada, a relatively new entrant, has rapidly become a major player, known for its ethically sourced, high-quality diamonds.
Other notable diamond-producing countries include South Africa (historically significant), Angola, Namibia, Zimbabwe, Tanzania, Sierra Leone, Lesotho, and Brazil. Each of these countries contributes unique characteristics to the global diamond market, from the large, high-quality stones of Lesotho to the colored diamonds of Brazil.
The global diamond industry extends beyond mining to cutting and polishing centers. The world’s major diamond processing hubs are:
Antwerp’s prominence stems from its centuries-old tradition, advanced cutting techniques, and the “Antwerp Cut” brand, which is synonymous with exceptional quality and precision in diamond cutting.
This global distribution of diamond production and processing reflects the industry’s complex supply chain, influenced by geological factors, technological advancements, economic policies, and shifting market demands.