
Have you ever wondered why steel sometimes fails unexpectedly? In this illuminating blog post, we’ll dive deep into the fascinating world of steel defects. As an experienced mechanical engineer, I’ll shed light on the various types of flaws that can compromise steel’s strength and performance. Discover the hidden causes behind these imperfections and learn how to identify and prevent them. Get ready to gain invaluable insights that will change the way you view steel forever!

Steel defects refer to various abnormal occurrences on the surface or inside of steel during its production or use that may impact its performance and quality.
Common surface defects in steel include cracks, scratches, folds, ears, scabs (heavy skin), welding scars, and end burrs. Additionally, there are typical surface defects like rolling oxides, patches, splits, pitted surfaces, and inclusions.
The causes of steel defects are diverse, such as severe damage or wear of the previous hole type roll groove, foreign metals falling on the rolled pieces and being pressed into the steel surface, or defects on the surface of the previous pass rolled piece. The oxidative atmosphere during heating also leads to steel oxidation, forming oxides such as FeO, Fe2O3, Fe3O4 on the workpiece surface.
Steel defect detection techniques mainly divide into traditional manual visual detection and automated detection based on computer vision. In recent years, methods based on deep learning, like YOLOv5 and YOLOv7, have been extensively applied in the automated detection of steel surface defects.
For certain specific defects, like banding, they can be eliminated through the method of high-temperature diffusion annealing. This process involves heating above 1050℃ to allow for uniform carbon atom diffusion, thereby eliminating banding.
Steel defects not only affect the physical properties of steel but may also present safety hazards during use. Therefore, the detection and treatment of steel defects are crucial to ensure steel quality and safe usage.

The specific reasons and mechanisms for the defects in steel mainly include the following points:
Surface defects: These defects include cracks, scratches, folds, ears, etc. The formation of cracks may be due to subsurface bubbles in the steel ingot, uncleaned cracks, and non-metallic inclusions that rupture or extend during rolling, as well as internal cracks in the steel ingot that expand and expose to the surface during rolling. In addition, factors such as inconsistent cooling conditions on both sides of the steel plate, uneven temperature of the rolled piece, uneven deformation during the rolling process, and uneven spray water cooling on the steel belt roller path can also cause surface defects.
Internal defects: These include shrinkage residuals, delamination, white spots, segregation, non-metallic inclusions, looseness, etc. These defects are mainly caused by equipment, process, and operation reasons during the steelmaking process.
Shape and size defects: These defects may involve size control issues during the production of steel. Although the specific generation mechanism is not detailed in the information I searched for, it can be inferred that it is related to temperature control, pressure distribution, and other factors during the production process.
Other factors: For example, deficiencies caused by equipment, process, and operation reasons during the smelting and rolling (forging) processing of carbon steel, including scabs, non-metallic inclusions, etc. In addition, the impact of irresistible factors such as material properties and processing technology in steel production may also cause different types of defects on the surface, such as rolling scales, spots, etc.
Materials form the foundation for the production of durable tools. During actual production, various types of material defects are frequently encountered.
Today, we will enlighten you on the 16 types of steel defects so that you are cautious when selecting raw materials.
After conducting an acid etching test on steel, it was discovered that some regions of the sample’s surface were not dense and displayed visible voids.
These voids, which appear as dark spots with uneven color shades compared to other areas, are known as porosity.
When the porosity is concentrated in the central part of the sample, it is referred to as central porosity, whereas if it is evenly distributed on the surface, it is called general porosity.
Both GB/T9943-2008 for High-Speed Tool Steel and GB/T1299-2014 for Tool Steel have specific regulations regarding the porosity of steel, but supplies often exceed the standard.
Porosity has a significant impact on steel strength, and its main hazards are as follows:
Since porosity affects the performance of steel, tool steel has stringent requirements for allowable porosity levels.
Figures 1 and 2 depict φ90mm W18Cr4V (abbreviated as W18) steel raw materials, showing porosity and porosity cracking patterns after a heat etching treatment with 1:1 HCl.
Figure 3 shows a picture of a W18Cr4V steel slotted milling cutter that suffered severe cracking due to sparing during heat treatment, as depicted through heat etching with 1:1HCl.

Figure 1 Central porosity

Figure 2 Cracks of central porosity steel during forging billets
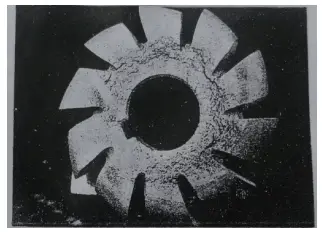
Figure 3 Cracks of slotting cutter material due to porosity during heat treatment
During the casting of an ingot, the liquid steel condenses and shrinks in the central part, forming a tubular hole known as shrinkage.
Typically, shrinkage is found near the feeder in the head of the ingot and should be removed when forming the billet.
However, the portion that cannot be completely removed is referred to as shrinkage residue.
While it is ideal to completely remove shrinkage, steel mills often prioritize production efficiency and leave a residue, resulting in irreversible consequences for subsequent processes.
Figure 4 shows φ70mm W18 steel with shrinkage residue and severe porosity, as depicted through heat etching with 1:1 HCl.
Figure 5 displays φ70mm W18 steel with shrinkage residue that has formed cracks after rolling, as depicted through heat etching with 1:1 HCl.
A few years ago, a company encountered shrinkage residue while sawing φ75mm M2 steel.
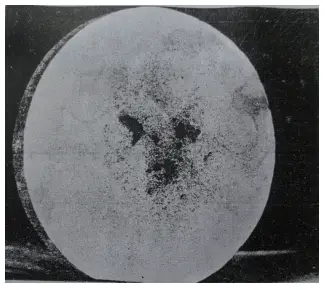
Figure 4
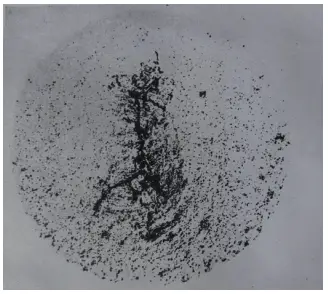
Figure 5: Cracks caused by W18 steel shrinkage
Longitudinal cracks on the surface of high-speed steel raw materials are a common occurrence.
There can be various causes for this, such as:
(1) During hot rolling, stress concentration may occur during the cooling process, leading to cracks along scratch lines due to incomplete removal of surface cracks or scratches caused by die holes.
(2) Poor die holes or large feed rates during hot rolling can lead to folds, which cause cracks along fold lines in subsequent processing.
(3) Cracks can be produced during hot rolling if the rolling stop temperature is too low or the cooling rate is too fast.
(4) Surface cracks are frequently observed on 13mm × 4.5mm W18 steel flat steel that is rolled in cold winter weather, indicating that the cracks may also be influenced by climatic conditions.
However, no cracks are observed when the same steel grade and specification is rolled at other times.
Figure 6 shows the surface crack of φ30mm W18 steel, with a depth of 6mm, as depicted through heat etching with 1:1 HCl.
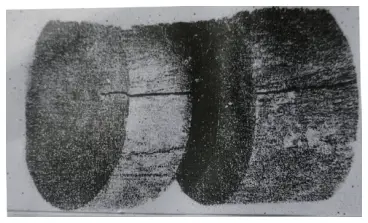
Figure 6 Surface crack
During the hot rolling process of high-speed steel, excessive deformation can cause the central temperature to increase rather than decrease. This can lead to the formation of cracks in the material center due to thermal stress.
Figure 7 shows the center crack in φ35mm W18 steel (etched with 1:1 HCl).
Central cracks in high-speed steel raw materials are common in tool plants, however, they are harmful as they are invisible and cannot be detected by touch. The only way to observe these cracks is through flaw detection.

Figure 7 Central crack
The uneven distribution of chemical elements within an alloy during the solidification process is known as segregation. This can have a significant impact on the performance of the steel, especially if there is an uneven distribution of impurities such as carbon.
Segregation can be further divided into microsegregation, density segregation, and regional segregation.
Density segregation occurs due to the differences in the density of constituent phases in the alloy, causing heavier elements to sink and lighter elements to float during solidification. Regional segregation is caused by the local accumulation of impurities in ingots or castings.
Figure 8 shows a quenched metallographic sample of W18 steel (etched using a 4% HNO3 alcohol solution), which reveals a cross-shaped pattern.
Further analysis of the chemical composition showed that the matrix part had a lower carbon content, while the cross-shaped part had a higher carbon content.
This cross-shape is a result of square segregation caused by the segregation of carbon and alloy components during the rolling process.
Serious regional segregation can weaken the strength of the steel and make it more susceptible to cracking during hot working.

Figure 8 Cross-shaped segregation (3×)
The extent to which the eutectic carbides in high-speed steel (HSS) break down during the hot press process is referred to as carbide nonuniformity. The greater the deformation, the higher the degree of carbide fracture and the lower the level of carbide nonuniformity.
When the carbides in the steel are severely broken down, such as in the form of coarse ribbons, meshes, or large carbide buildup, it has a significant impact on the quality of the steel. It is therefore crucial to carefully control carbide nonuniformity to ensure the quality of HSS tools.
Figure 9 depicts the effect of carbide nonuniformity on the bending strength of W18 steel.
As can be seen from the figure, the bending strength in grades 7-8 with nonuniformity is only 40-50% of grades 1-2, reducing the strength to 1200-1500MPa, which is only equivalent to the level of higher toughness grades in cemented carbides. The horizontal performance is around 85% of the vertical performance.
The concentration and band-like distribution of carbides can also result in uneven quenched grains and uneven dissolution of carbides, leading to an increased tendency of overheating and a reduction in secondary hardening ability, respectively.
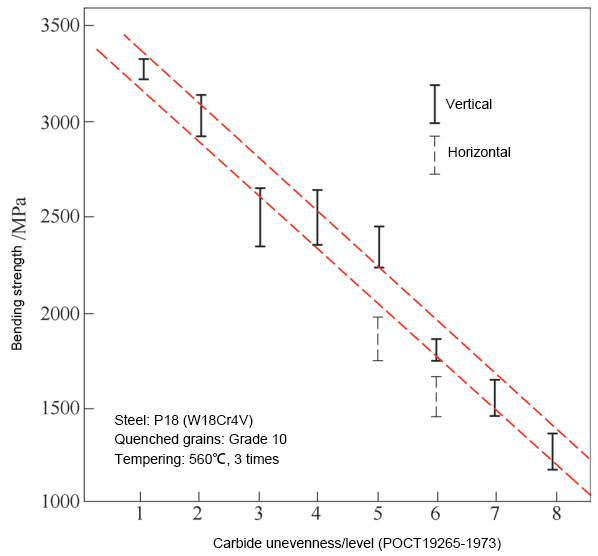
Figure 9 depicts the impact of carbide nonuniformity on the bending strength of W18Cr4V high-speed steel.
It can be seen that severe carbide nonuniformity can result in cracking and overheating during hot working, causing the finished tool to fail in use.
Figure 10 illustrates the quenching crack caused by coarse zonal carbides in W18 steel (etched with a 4% HNO3 alcohol solution).

Figure 10 Coarse zonal carbide
Steel that has undergone hot rolling or annealing can form network carbides due to high heating temperatures, extended holding times that cause grain growth, and slow cooling processes that result in carbide precipitation along grain boundaries.
The presence of network carbides greatly increases the brittleness of the tool, making it more prone to chipping. In general, complete network carbides are not acceptable in steel.
The inspection for network carbides should be conducted after quenching and tempering.
Figure 11 shows the network carbides of T12A steel (etched with 4% HNO3 alcohol solution), while Figure 12 shows the morphology of the network carbides of 9SiCr steel (etched with 4% HNO3 alcohol solution), revealing severe overheating during the annealing process.

Figure 11 T12A Steel Mesh Carbide (500×)

Figure 12 9SiCr Steel Mesh Carbide (500×)
Tool mills that perform HSS turning or milling may encounter a hard substance and sustain damage. This defect is typically not easily found during high-speed turning, due to the high cutting speed and noise.
However, during milling, lumps and strange chaos may be observed, such as a squeaking sound and severe burnout of the tool when milling slots with twist drills.
Upon inspection, bright blocks can be seen with the naked eye and have been found to have extremely high hardness, reaching 1225HV, while the non-hard areas are in a normal annealing state. This is referred to as a “caked mass”.
The presence of caked masses results in tool damage and makes cutting difficult.
The formation of these hard lumps is thought to be caused by the segregation of chemical components during the smelting process and may be a kind of high-hardness composite carbide or the result of the addition of refractory alloy blocks during smelting.
Figure 13 shows the macrostructure of a caked mass in W18 steel (etched by 4% HNO3 alcohol solution), with the white substance being the caked mass and the gray and black areas representing the bit grooves.

Figure 13 The macrostructure of W18 steel caked mass (20×)
Inclusions are a common defect in steel that can be classified into two categories: metallic inclusions and non-metallic inclusions.
Metallic inclusions form due to the incomplete melting of ferroalloy during the smelting process or the presence of foreign metal particles that remain in the steel ingot.
Non-metallic inclusions are divided into two types:
(1) endogenous inclusions, which are mainly caused by dirty pouring systems, peeling refractory mud from equipment, or using impure charge materials;
(2) inclusions produced and precipitated due to chemical reactions during the smelting process. Figure 14 shows metal inclusions found in W18 steel, while Figure 15 depicts non-metallic inclusions causing cracks during quenching (etched with 4% HNO3 alcohol solution).
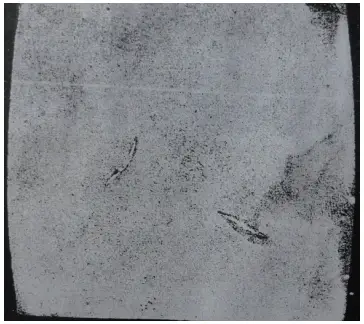
Figure 14 Metal inclusions

Figure 15 Cracking caused by non-metallic inclusions during quenching (400 x)
Inclusions are detrimental to the quality of steel. They segment the steel matrix, decrease its plasticity and strength, making the steel susceptible to cracking around the inclusions during rolling, forging, and heat treatment.
Inclusions can also cause fatigue in the steel, as well as difficulties during cutting and grinding. Therefore, tool steel must have specified requirements for inclusions.
In the process of steel smelting, uneven distribution of carbides can occur due to component segregation, or when carbides in the iron alloy are not fully melted, resulting in large angular carbides that persist without being crushed after forging.
The presence of these bulk carbides increases the tool’s brittleness and increases the risk of tipping.
During the heat treatment process, these large carbides and alloying elements can become enriched, potentially leading to defects such as overheating, insufficient tempering, and even cracking along grain boundaries.
Figure 16 shows overheating during quenching caused by segregation of surrounding components of large carbides (etched in 4% HNO3 alcohol solution).
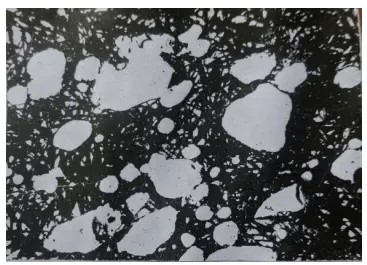
Figure 16 Overheating caused by segregation of components around bulk carbides during quenching (500×)
In the solidification process of liquid metal, segregation of carbon and alloying elements can cause large blocks of carbide to precipitate during cooling.
This segregation, known as liquation, is not easily eliminated during subsequent processing and results in the presence of bulk zoster carbide in the direction of the rolling of the steel.
Figure 17 shows CrMn liquation, as etched with a 4% HNO3 alcohol solution.

Figure 17 Carbide liquation (500×)
Steels with liquation are highly brittle, as the continuous metal matrix is disrupted, resulting in reduced strength. Previously, liquation was commonly found in CrWMn and CrMn steels, and using them to make gauges often resulted in difficulty in obtaining a smooth surface.
As the annealing temperature is too high and the holding time is too long, during the slow cooling process of the steel, carbides easily decompose into free carbon, known as graphite.
Figure 18 shows the microstructure of graphite carbon in T12A steel (etched with 4% bitter acid alcohol solution).

Figure 18 Graphitic carbon microstructure of T12A steel (500×)
The precipitation of graphite carbon significantly decreases the strength and toughness of steel, making it unsuitable for the production of knives and critical components. The steel exhibits black fractures when it contains high levels of graphite carbon.
The presence of graphite carbon can be determined through chemical analysis for both qualitative and quantitative analysis, and its shape and distribution can be observed through metallographic methods.
Additionally, there will be an increase in ferrite tissue around the graphite.
Mixing of materials in tool and mold manufacturing enterprises is a common problem, a result of poor management and a low-level defect. The mixed materials can include three aspects: mixed steel, mixed specifications, and mixed furnace numbers.
The last one is especially prevalent and can cause many issues with false heat treatments with no way to appeal. From time to time, unqualified tool material components are also encountered.
Some high-speed steel components do not meet the GB/T9943-2008 High-speed Tool Steel standard, especially regarding the high or low content of carbon. For example, W6Mo5Cr4V2Co5 belongs to the HSS-E type but has a lower carbon content than the standard lower limit.
Despite being labeled as high-performance HSS, after heat treatment, the hardness does not reach 67HRC. Steel mills must ensure that the steel can reach a hardness of at least 67HRC if they belong to the HSS-E type.
Whether a tool requires such high hardness is an internal matter for the tool factory and is not the responsibility of the steel mill.
However, if the hardness does not reach 67HRC, it is a fault of the steel mill. There are also many cases of unqualified die steel composition, leading to ongoing disputes.
The country has established standards for the decarburization of steel, however, steel suppliers often supply materials that exceed these standards, resulting in significant economic losses for tool manufacturing companies.
The surface hardness of tools decreases and their wear resistance is poor after quenching for materials with a decarburized layer. Therefore, it is necessary to completely remove the decarburized layer during machining to avoid any potential quality problems.
Figure 19 illustrates the decarburization morphology of W18 steel raw material (etched in 4% HNO3 alcohol solution). The decarburization zone is needle-shaped tempered martensite, while the non-decarburized zone is composed of quenched martensite, carbides, and retained austenite.
Figures 20 and 21 show the decarburization of M2 and T12 steel, respectively (etched in 4% HNO3 alcohol solution).
In the case of T12 steel, the fully decarburized layer is ferrite, the transition zone is composed of carbon-lean tempered martensite, and the non-decarburized zone is composed of tempered martensite and carbides.

Figure 19 Austempered decarburization layer (250×)
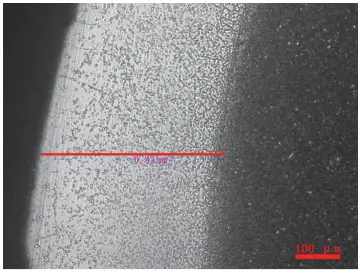
Figure 20 Decarburization of M2 steel

Figure 21 Decarburized layer of T12A steel (after quenching→tempering) (200×)
We selected a W18 steel flat bar with dimensions of 13mm x 4.5mm from a specific company and quenched it in a salt bath at temperatures of 1210℃, 1230℃, and 1270℃.
The heating time was 200 seconds, and the grain size was 10.5, as shown in Figure 22. The hardness after quenching was between 65 and 65.5HRC, but surprisingly, the hardness decreased after tempering at 550℃ for three times.
This anomaly is referred to as “an anecdote.
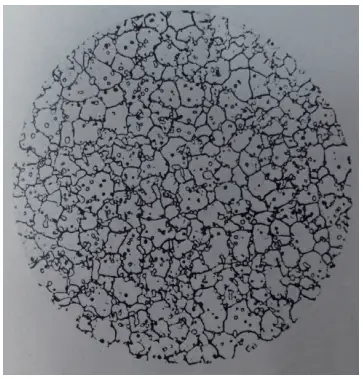
Figure 22 W18 steel quenching Grade 10.5 (500×)
It appears that the carbide is playing a trick on us, meaning that when the carbide is heated, it does not dissolve into austenite nor precipitate during the tempering process.
This is simply referred to as not being able to get in or out, so where’s the secondary hardening?
The root of the issue is that the carbide is teasing us, meaning that it doesn’t dissolve into the austenite during heating and there is no precipitation during the tempering process.
It’s simply a case of not being able to get in or out, so where’s the secondary hardening coming from?
Surface defects are easily visible with the naked eye, such as:
Steel products can be affected by various defects that fall into different categories, each with specific characteristics and implications for the material’s quality and performance. Understanding these defects is essential for maintaining the integrity and reliability of steel products across different applications.
Roll marks, also known as bruises or whip marks, appear as indentations or raised areas on the surface of steel sheets and thicker-walled products like billets. These defects are typically caused by irregularities or debris on the rolling mill’s surface. For instance, in the automotive industry, roll marks can affect the surface finish of car body panels, leading to additional processing costs.
Overlaps occur when excessive filler material is used during welding, resulting in uneven surfaces or material hanging down from the specimen. This defect can affect both the appearance and workability of the steel. In pipeline construction, overlaps can lead to difficulties in achieving a smooth surface finish, complicating the welding process.
Seams are linear imperfections that form during the rolling or forging process. They appear as visible lines on the steel’s surface and can compromise the material’s structural integrity. For example, in the aerospace industry, seams in structural components can reduce fatigue life and lead to premature failure.
Laminations are internal separations within the steel’s layers, often resulting from improper rolling or forging techniques. These defects can significantly weaken the steel, making it prone to failure under stress. In the construction of pressure vessels, laminations can lead to catastrophic failures due to the high pressures involved.
Scabs are raised or depressed areas on the steel surface caused by improper rolling or forging, while laps occur when two layers of steel overlap, creating a raised area. Both defects can weaken the steel and affect its surface finish. In manufacturing, scabs and laps can lead to rejection of products due to poor aesthetics and compromised mechanical properties.
Inclusions are non-metallic particles trapped within the steel during manufacturing. These particles, such as slag or oxides, create weak points that can initiate cracks and reduce the material’s overall strength. In high-stress applications like bridge construction, inclusions can lead to structural failures.
Porosity refers to the presence of small voids or cavities within the steel, often resulting from improper casting or welding techniques. These voids compromise the steel’s ductility and corrosion resistance. In marine environments, porous steel can suffer from accelerated corrosion, reducing the lifespan of ship hulls.
Segregation occurs when alloying elements in the steel are unevenly distributed during solidification, leading to variations in the material’s composition and mechanical properties. This defect can result in inconsistent performance, especially in structural components. For example, in heavy machinery, segregation can lead to uneven wear and unexpected failures.
Quench cracking happens during the quenching process, where rapid cooling induces cracks on the steel’s surface. This defect is common in alloys like 4140 chrome steel billets and can severely compromise the material’s strength and durability. In tools and dies, quench cracks can lead to early breakage and reduced tool life.
Pinchers are uneven ridges or grooves that appear on steel products, particularly those that are rolled, such as billets, pipes, or tubes. While minor pinchers may not significantly affect the product’s quality, prominent ones can necessitate adjustments in the manufacturing process. In oil and gas pipelines, pinchers can create points of stress concentration, leading to potential leaks.
Deformations, such as bending, twisting, or warping, can occur due to improper handling, excessive loading, or thermal stresses. These defects compromise the structural integrity and performance of steel products. In construction, deformed steel beams can lead to misalignment and structural instability.
Cold shut defects are rounded-edge cracks that occur when two streams of molten steel do not fuse properly during casting. This defect can arise from improper gating system design, low temperatures of the liquid metal, or reduced fluidity of the molten metal. Cold shuts weaken the steel and can lead to failures in critical applications, such as engine components.
Unfilled sections occur when some parts of the forging remain unfilled, often due to poor die design, insufficient raw material, or improper heating. This defect results in incomplete or weak areas within the forged product. In aerospace components, unfilled sections can lead to structural weaknesses and potential in-flight failures.
Scale pits are irregular depositions on the surface of forgings, typically caused by improper cleaning of the forged surface. These pits can be removed through proper cleaning methods to restore the smoothness and quality of the forged product. In high-precision industries, such as medical implants, scale pits can lead to product rejection.
Die shift happens when the upper and lower dies are not properly aligned during forging, resulting in products with incorrect dimensions. Ensuring proper die alignment can correct this defect. In automotive parts, die shift can cause issues with assembly and fitting.
Flakes are internal cracks that occur due to improper cooling of the forged product. Rapid cooling can induce these cracks, reducing the strength and reliability of the forging. In high-pressure applications like hydraulic systems, flakes can lead to sudden failures.
Residual stresses develop due to improper cooling of the forged part, particularly if the cooling is too rapid. These stresses can lead to distortions and potential failure of the forged product. In structural components, residual stresses can cause warping and misalignment, affecting overall stability and performance.
Understanding these specific types of steel defects is crucial for quality control, ensuring the reliability and longevity of steel products, and preventing potential failures in critical applications.
The manufacturing processes of steel products involve various stages, each with potential defects that can compromise the final product’s quality and performance. Understanding these defects and their origins is crucial for implementing effective quality control measures and improving manufacturing efficiency.
Visual inspection is a critical technique in identifying defects in steel products, ensuring quality control, and preventing potential failures. This process involves various traditional and advanced methods to detect surface and subsurface imperfections.
Direct visual inspection involves examining the steel surface with the naked eye. Inspectors look for visible defects such as cracks, seams, and surface imperfections. For instance, a longitudinal crack in a steel beam can compromise its structural integrity, leading to catastrophic failures. This method is flexible and cost-effective, but its reliability depends on the inspector’s skill and experience. Additionally, it is limited to detecting defects on accessible surfaces and may miss flaws in hard-to-reach areas.
Industrial microscopes, often equipped with cameras and image analysis software, enhance the detection of minute defects not visible to the naked eye. These microscopes can identify fine cracks, inclusions, and other small imperfections that could lead to material failure. For example, the presence of non-metallic inclusions can weaken steel, affecting its mechanical properties. However, the effectiveness of this method can be influenced by the operator’s proficiency and the sample size.
Borescopes are instruments equipped with a camera and a light source, allowing inspection of internal areas that are not visually accessible, such as inside castings or complex assemblies. For example, a borescope can reveal internal voids or cracks in a welded joint that could lead to fatigue failure under cyclic loading. Borescopes provide a detailed view of internal surfaces, making it possible to detect defects that traditional visual methods would overlook.
MT involves magnetizing the steel part and applying ferrous particles. These particles are attracted to areas of flux leakage, indicating the presence of surface or subsurface defects. Flux leakage occurs when there is a disruption in the magnetic field, which can be caused by cracks or other discontinuities. MT is relatively simple and inexpensive but is limited to ferromagnetic materials.
In PT, a liquid penetrant is applied to the steel surface, which seeps into cracks and crevices. After a dwell time, the excess liquid is removed, and a developer is applied, making the penetrant visible under ultraviolet light. This method is widely used for detecting surface-breaking defects in non-porous materials, such as aluminum or stainless steel.
UT employs high-frequency sound waves to detect internal defects such as cracks, voids, and inclusions. The sound waves travel through the steel, and any disruption in their pattern indicates the presence of defects. For example, a longitudinal flaw in a steel pipe can be detected through UT by observing changes in the reflected sound waves. UT is highly effective for identifying subsurface imperfections.
RT uses X-rays or gamma rays to create an image of the internal structure of the steel. Defects appear as areas with different densities compared to the surrounding material. RT is particularly useful for detecting internal defects that are not visible on the surface, such as voids in welds or inclusions within castings.
ECT detects surface and subsurface defects through electromagnetic induction. An alternating current is passed through a coil, generating an electromagnetic field. When a conductive material, such as steel, is introduced, eddy currents are induced within the material. A defect disturbs the flow of these eddy currents, measurable by variations in impedance in the coil. This method is especially effective for detecting surface-breaking cracks in steel welds.
AOI systems utilize high-resolution cameras, sophisticated lighting, and software algorithms to perform rapid, objective, and accurate surface inspections. These systems can process thousands of images in a short time, significantly enhancing the efficiency of the inspection process and ensuring consistent quality control.
Various machine learning and deep learning techniques are employed to detect and classify surface defects in steel products. Techniques such as artificial neural networks and deep learning models enhance accuracy and efficiency. For instance, a deep learning model trained on a large dataset of defect images can automatically identify and categorize defects, reducing the reliance on human inspectors and improving inspection speed.
Identifying steel defects through visual inspection is a comprehensive process that combines traditional methods, advanced technologies, and innovative techniques. Each method has its advantages and limitations, and the choice depends on the specific application, defect type, and material properties. By integrating these techniques, industries can ensure higher quality and safety standards for steel products.
Steel defects can arise from various factors related to manufacturing processes, material properties, and environmental conditions. Understanding these causes is essential for identifying potential sources of defects and implementing preventive measures to ensure the quality and integrity of steel products.
In summary, steel defects can stem from various sources, including manufacturing processes, material properties, and environmental conditions. Addressing these causes through quality control measures, material selection, and appropriate manufacturing practices is essential for preventing the occurrence of defects and maintaining the quality and reliability of steel products.
Preventing steel defects in manufacturing is crucial for maintaining product quality and ensuring production efficiency. Steel defects can lead to significant financial losses, compromise structural integrity, and negatively impact customer satisfaction. This chapter provides a comprehensive approach to minimizing steel defects through process control, quality assurance, advanced technologies, and meticulous material handling.
Optimizing the manufacturing process is essential to prevent defects. A clean and well-ventilated workspace helps prevent scale formation and other surface issues. Regular inspections can identify potential problems early, allowing for prompt corrective actions. Proper handling techniques and protective coatings are vital to avoid surface damage such as scratches and dents. Utilizing padded supports and careful handling can significantly reduce the risk of surface imperfections.
Temperature control is crucial in preventing defects like warping, cracking, and surface peeling. For instance, in welding and cutting processes, controlling the heat input is vital. Techniques like preheating or post-weld heat treatment can help prevent warping. In galvanizing processes, avoiding overheating the zinc and maintaining correct aluminum levels in the galvanizing bath can prevent surface peeling or flaking. For example, a case study in a steel mill showed that precise temperature control during galvanizing reduced surface defects by 30%.
Choosing the right materials and preparing them properly can reduce defects. Selecting the appropriate material thickness and ensuring steady cooling rates can help prevent warping and other dimensional issues. Using clean, low-impurity source metals is essential to avoid slag inclusions and other casting defects. Reducing elements that react strongly with oxygen further minimizes the risk of inclusions. Slag inclusions, for example, are non-metallic particles trapped within the metal during the casting process. Using low-impurity metals can significantly reduce these inclusions.
Employing trained engineers with experience in metal manufacturing enhances the production process. Engineers can design products with fewer operational and design errors, which can be identified and corrected early using smart technology. Implementing robust quality control measures throughout the creation process is essential. Regular inspections and maintenance of molds and equipment help identify and prevent defects such as mold material defects and slag inclusions.
Advanced technologies during inspections can help identify potential issues early. Image scanners and computer programs detect operational and design errors before production, allowing for timely corrections. Simulation technologies like THERCAST®, which simulates casting processes, can optimize casting parameters without actual metal pouring, reducing the risk of defects like slag inclusions.
Utilizing precise cutting methods improves the dimensional accuracy of steel products. CNC cutting and laser cutting can reduce inaccuracies and burr formation. Automated deburring tools smooth out rough edges, enhancing the final product quality. CNC (Computer Numerical Control) cutting uses computer software to control cutting tools, ensuring high precision and consistency.
Preventing red rust and staining involves ensuring there are no leaks in the rinse tank and maintaining proper accumulator conditions. Regular cleaning and maintenance of the production line can help avoid staining and other surface defects. For instance, a steel manufacturer reduced red rust incidents by 25% after implementing a stringent cleaning schedule.
To prevent slivers and laps, using the correct rolling processes and ensuring defects are removed by conditioning is crucial. Ensuring segment rolls at the continuous caster are not locked up helps avoid laps. A case study in a rolling mill showed that optimizing the rolling process reduced slivers by 15%.
Preventing slag inclusions involves optimizing pouring temperature and rate. Reducing turbulence during the pour and ensuring the mold cavity is clean helps prevent inclusions. Implementing slag traps and a full pouring basin further minimizes the risk of inclusions. Slag inclusions are often caused by impurities that are not removed during the casting process. Using slag traps can effectively capture these impurities, improving the final product quality.
By integrating these strategies, manufacturers can significantly reduce the occurrence of steel defects, ensuring higher quality products and improved production efficiency.
Addressing and remedying steel defects requires a thorough understanding of the specific types of defects, their causes, and the appropriate corrective actions. Here are detailed solutions and remedies for common steel defects encountered in various manufacturing processes, supported by specific examples and case studies to illustrate the concepts more clearly.
Shrinkage defects occur due to uneven metal shrinkage, resulting in dips or holes on the surface.
Example: A foundry experienced significant open shrinkage defects in their cast iron products. By redesigning the gating system to improve metal flow and increasing the pouring temperature, they reduced these defects by 40%.
Hot tears result from thermal contractions and improper solidification processes.
Example: A steel mill faced hot tear issues in large castings. By using fillets at junctions and accurately placing gates, they minimized stress concentrations, reducing hot tears by 30%.
Cold shuts arise from improper gating system design and low metal temperatures.
Example: A manufacturer eliminated cold shuts in aluminum castings by optimizing the gating system and increasing the pouring temperature, leading to a 25% improvement in product quality.
Soldering defects include damages to the die cavity and improper temperatures.
Example: A company faced soldering defects in zinc die casting. By using high-quality mold-release agents and ensuring adequate iron content in the alloy, they improved surface finish and reduced defects by 20%.
Cracks occur in areas of compression, particularly in rectangular draw shapes.
Example: An automotive manufacturer reduced cracks in fender panels by stress relieving the material before operations, achieving a 15% reduction in defects.
Wall thinning results from material deformation during forming processes.
Example: A supplier improved the thickness uniformity of metal tubes by using materials with higher R-values and adjusting forming parameters, enhancing product consistency by 20%.
Burrs are caused by dull cutting tools and improper tool alignment.
Example: A precision engineering firm reduced burr formation in machined parts by resharpening cutting tools regularly and maintaining proper tool alignment, leading to a 30% reduction in post-processing time.
Splitting occurs when the material’s ultimate tensile strength is exceeded.
Example: A sheet metal fabricator reduced splitting in deep-drawn parts by reducing strain in high-strain areas and increasing stretch in the minor direction, improving yield by 10%.
Wrinkles arise from areas of compression inhibiting material flow.
Example: A metal forming company minimized wrinkling in large panels by reducing compressive forces in corners and using materials with higher R-values, achieving a smoother finish.
Springback results from coil camber or improper tooling sensitivity.
Example: A coil processing plant eliminated springback issues by ordering coils slit to eliminate cambering and adjusting tooling sensitivity, enhancing product accuracy.
Surface peeling or flaking occurs due to poor adhesion to steel and overheating.
Example: A galvanizing plant improved adhesion and reduced peeling by maintaining optimal bath temperature and aluminum levels, increasing product lifespan by 15%.
Rust forms due to moisture exposure and poor storage conditions.
Example: A steel distributor reduced rust formation by maintaining a clean, well-ventilated workspace and ensuring proper storage conditions, improving product quality.
For parts not meeting specifications, consider re-milling, grinding, or hand-finishing to correct defects and achieve the desired results. These corrective actions can restore the integrity and functionality of steel products, ensuring they meet quality standards.
Below are answers to some frequently asked questions:
Steel products can suffer from various defects that impact their quality, performance, and safety. Common defects can be classified into surface defects, internal defects, and mechanical defects.
Surface defects include cracks, which can lead to catastrophic failures and may result from improper heat treatment, welding, or mechanical stress. Seams are linear imperfections formed during rolling or forging, weakening the steel. Laminations occur when layers of steel separate, often due to improper rolling or forging techniques. Scabs are raised or depressed areas on the surface caused by improper rolling or forging, affecting the steel’s strength and finish. Laps are overlapping layers of steel that create raised areas, weakening the steel. Other surface imperfections like pits, scales, and inclusions can negatively impact the appearance and performance of the steel. Edge irregularities, such as wave-like patterns, can also occur.
Internal defects include inclusions, which are non-metallic particles trapped in the steel during manufacturing, weakening the material and increasing the risk of cracks. Porosity refers to small voids or cavities within the steel that compromise its ductility and corrosion resistance, often due to improper casting or welding. Segregation involves the uneven distribution of alloying elements, leading to variations in the material’s composition and mechanical properties.
Mechanical defects encompass deformations like bending, twisting, or warping caused by improper handling, excessive loading, or thermal stresses, compromising structural integrity. Misalignments arise from inaccurate assembly or poor fabrication practices, leading to stress concentrations and potential failure points. Welding defects, such as porosity, incomplete fusion, cracks, undercuts, and excessive spatter, can compromise weld integrity.
Other defects include dimensional deviations like warping, bowing, twisting, or variations in length or width, affecting structural integrity and complicating installation. Material contamination with foreign substances like oil, dirt, or paint can affect weld integrity and coating adhesion. Inadequate reinforcement, such as insufficient rebar or mesh, can lead to structural weaknesses like cracks or collapse. Coating adhesion issues may expose the substrate due to material impurities. Surface imperfections in flat-roll steel, such as wavy patterns, streaking, raised spots, and surface ripples or creases, can result from handling or manufacturing damage.
Addressing these defects through quality control, process optimization, and effective training of personnel is crucial for ensuring the reliability and longevity of steel products.
Visual inspection is a fundamental method for identifying defects in steel, leveraging both traditional and advanced techniques to ensure thorough examination. Traditional visual inspection involves direct examination using the naked eye, which is cost-effective and flexible but can be inconsistent due to variations in inspector skill and limitations in inspecting low-visibility areas.
To address these limitations, enhanced visual inspection techniques are used. Light enhancement techniques, such as angle and diffuse lighting, help highlight surface irregularities by creating shadows or providing clear views, respectively. Fluorescent Penetrant Inspection (FPI) uses fluorescent liquids that seep into cracks, glowing under ultraviolet light to reveal defects.
Microscopic inspection employs industrial microscopes with cameras and image analysis software to detect minute defects, offering a non-destructive testing method, although it may require skilled operators. Borescope inspection is useful for examining internal structures, providing detailed views of areas not visible to the naked eye.
Automated Optical Inspection (AOI) systems use high-resolution cameras, sophisticated lighting, and software algorithms for fast, objective, and accurate surface inspections. These systems benefit from continuous improvement through AI and machine learning, enhancing defect identification and predictive maintenance.
Integrating machine learning and AI into vision-based methods significantly improves defect detection and classification. Models like Artificial Neural Networks and Deep Learning handle large datasets and diverse defect types efficiently. Standardization and benchmarking are crucial for comparing algorithms, while data augmentation enhances model performance. Human expertise remains vital in refining and validating these models, especially with small datasets.
By combining these visual inspection techniques with advanced technologies, industries can achieve more accurate and efficient defect detection in steel surfaces.
The main causes of defects in steel can be attributed to several factors, which can be broadly categorized into material-related factors, process-related issues, and environmental conditions.
Material-related factors include the presence of inclusions and segregation. Inclusions are non-metallic particles such as slag and oxides that can weaken the steel, resulting from incomplete melting of materials or contamination during casting. Segregation occurs when elements like manganese, carbon, and sulfur concentrate unevenly during casting, causing local variations in composition that can lead to defects like white spots and cracks.
Process-related issues encompass improper heating and cooling, poor die design and alignment, and inadequate forging and rolling techniques. Rapid or uneven cooling can induce defects such as flakes and surface cracking, while slow cooling can help mitigate these issues. Misaligned or poorly designed dies can lead to unfilled sections and die shifts. Deficient forging or rolling techniques can cause cracks, laminations, and surface irregularities due to uneven deformation and internal stress. Additionally, improper welding and heat treatment can result in cracks and porosity, with the skill and quality of the weld metal being crucial.
Environmental and operational factors also play a significant role. Improper surface cleaning and oxidative atmospheres during heating can lead to scale pits and oxidation, forming various oxides. Equipment wear and damage, such as in rolling grooves, and improper use of casting powder can cause surface and internal defects. Uneven temperature and pressure during production can result in longitudinal, transverse, and corner cracks in continuous cast steel products.
Other contributing factors include excessive mechanical stress during processing, leading to surface cracking and internal tearing, often exacerbated by inconsistent cooling and uneven deformation. Weather conditions, such as cold temperatures, can also affect the formation of cracks during rolling processes.
By understanding these causes, manufacturers can implement measures to minimize defects and ensure the quality and integrity of steel products.
Manufacturers can prevent defects in steel products through several key strategies implemented at various stages of the production process. Firstly, ensuring the quality of raw materials is crucial. This involves a robust incoming material inspection process to verify dimensions, standards, and grades, along with clear communication and accountability with suppliers.
Regular maintenance of equipment is vital to prevent defects, including both preventative and breakdown maintenance. Routine checks and maintenance ensure equipment operates correctly, reducing the risk of defects. For example, cleaning welding equipment and sharpening CNC machine blades can significantly reduce defects.
In casting processes, proper mold design and preparation are essential. This includes ensuring adequate ventilation to prevent gas entrapment, using high-quality mold materials, and controlling casting parameters such as temperature and pouring speed. Regular maintenance and inspection of molds are necessary to prevent wear and degradation.
Careful management of melting and pouring practices is necessary to prevent casting defects. Controlling pouring temperature, using techniques like vacuum-assisted casting, and modifying the chemical composition of molten material can reduce issues like gas porosity. Proper gating setups facilitate uniform cooling and solidification, preventing defects like cold shuts and misruns.
Surface preparation and protection are important to prevent surface defects. Maintaining a clean and well-ventilated workspace, using high-quality materials, and ensuring proper adhesion in processes like galvanizing are essential. Regular inspection during production, storage, and transportation helps identify potential problems early.
Implementing standard work instructions ensures operators know how to perform tasks and the expected outcomes, reducing guesswork. Quality control measures, such as using coordinate measuring machines or vision cameras, help identify defects early and maintain production efficiency.
Proper handling and storage of materials can prevent defects. Using high-strength, small grain size, low permeability sand and hard ramming of sand prevents metal penetration. Drying molds and cores before use and storing them dry prevents gas porosity.
Controlling the cooling and solidification process is critical. Factoring in shrinkage allowances into pattern design and increasing local heat dissipation with internal chills, cooling ribs, or coils can prevent shrinkage cavities.
By implementing these strategies, manufacturers can significantly reduce defects in steel products, ensuring higher quality and reliability in the final products.
Repairing steel defects involves several methodologies and considerations. Weld repair is suitable for addressing minor defects, salvaging components, and making prototype modifications. Defect removal, preheating, and proper welding techniques are crucial for effective repairs. Non-destructive testing should be conducted to ensure weld integrity. For critical applications, recasting may be necessary. Structural steel repair involves visual inspection, welding, bolting, and strengthening techniques to maintain integrity and functionality.
The specific impacts of steel defects on the physical properties of steel primarily include the following aspects:
Changes in hardness and plasticity: Influenced by certain factors, the strength of steel may increase, but at the same time, plasticity and toughness decrease, resulting in increased brittleness, a phenomenon known as hardening. This typically occurs under repeated loads, when the elastic limit increases and enters the plastic stage.
Effects on wear resistance and fatigue resistance: Surface quality defects not only affect the aesthetic appearance of hot-rolled strip steel, but can also have adverse effects on its mechanical properties and corrosion resistance, including wear and fatigue resistance.
Tool wear and unsmooth surfaces: The presence of looseness in the material can lead to excessive wear and unsmooth surfaces of the tools made from it. Hence, tool steel has strict requirements for the acceptable level of looseness.
Dispersion of microstructure and defects: The toughness of steel primarily depends on the dispersion of the microstructure and defects (avoiding concentrated defects), rather than the chemical composition. The toughness undergoes significant changes after heat treatment.
Effects of annealing and normalizing treatment: Annealing can reduce the hardness of steel, improve plasticity, refine grains, eliminate structural defects caused by casting, forging, and welding, homogenize the structure and composition of the steel, and relieve internal stress and work hardening in the steel. Normalizing has similar effects on large castings, forgings, and weldments.








