
Why do some metal objects stay shiny and rust-free while others quickly lose their luster? The secret lies in the type of plating used. This article explores the differences between chrome, nickel, and zinc plating, explaining their unique properties, applications, and costs. By the end, you’ll understand how each type of plating affects durability, appearance, and corrosion resistance, helping you make informed choices for your projects.

In industrial design, the electroplating process is frequently used. Firstly, let’s understand what electroplating is. Electroplating is the process of plating a thin layer of other metals or alloys onto the surface of some metals using the principle of electrolysis.
This process uses electrolysis to attach a metal film to the surface of metal or other materials to prevent metal oxidation (such as rusting), which improves wear resistance, conductivity, light reflection, corrosion resistance (such as copper sulfate, etc.), aesthetics, and other functions.
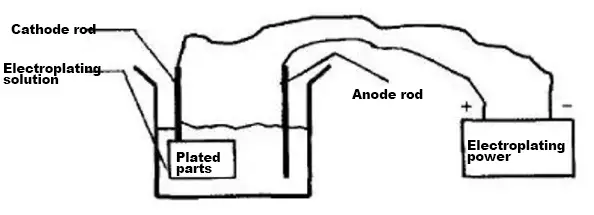
Schematic diagram of the electroplating process
Electroplating is divided into specific processes such as copper, gold, silver, chromium, nickel, and zinc plating. In the field of industrial design, especially galvanizing, nickel plating, and chromium plating are the most widely used. There must be some differences between these three methods, right?
Definition:
Zinc plating, also known as zinc electroplating or electrogalvanizing, is a surface treatment process that deposits a thin layer of zinc onto the surface of metal substrates, primarily steel or iron. This electrochemical process enhances the substrate’s corrosion resistance, improves its aesthetic appearance, and provides a sacrificial barrier against oxidation.
Features:
Applications:
Zinc plating is widely used across various industries due to its balance of performance, cost-effectiveness, and versatility in protecting ferrous metals from corrosion in moderately aggressive environments.

White zinc

Color zinc
Definition:
Nickel plating is a sophisticated surface treatment process that involves depositing a layer of nickel onto metallic or specific non-metallic substrates through electrochemical or chemical reduction methods. This technique, also known as electroplating when performed electrolytically, creates a durable and aesthetically pleasing finish.
Features:
Considerations:
Applications:
By leveraging its unique combination of functional and aesthetic properties, nickel plating continues to play a crucial role in enhancing the performance and appearance of a wide range of products across multiple industries.


Definition:
Chromium is a hard, lustrous, steel-gray metal with a bluish tinge, known for its excellent corrosion resistance and high melting point.
Chromium plating, also known as chrome plating or chromium electroplating, is an electrochemical process that deposits a thin layer of chromium onto a substrate material, typically metal or certain plastics, to enhance its surface properties.
Features: Chrome plating is categorized into two main types:
Applications:
Chrome plating has widespread use across various industries due to its unique combination of aesthetic appeal and functional benefits. However, environmental concerns regarding hexavalent chromium have led to the development of alternative processes, such as trivalent chromium plating and physical vapor deposition (PVD) coatings, which are gaining traction in certain applications.





Chromium plating is primarily employed to enhance surface hardness, improve aesthetic appeal, and provide corrosion resistance. The chromium coating exhibits excellent chemical stability, resisting most alkalis, sulfides, nitric acids, and organic acids. However, it can be dissolved in hydrochloric acid and hot sulfuric acid. Chromium’s superior color retention and reflective properties make it preferable to silver and nickel for long-term applications. The process typically involves electrodeposition, where chromium ions are reduced to metallic chromium on the substrate surface.
Nickel plating offers a combination of wear resistance, corrosion protection, and aesthetic enhancement. It is usually applied in thin layers (typically 5-50 μm) through either electroplating or electroless (chemical) deposition methods. Nickel’s versatility makes it suitable for both decorative and functional applications across various industries.
Zinc plating, often referred to as galvanizing, primarily focuses on corrosion protection and improving appearance. Zinc’s sacrificial nature, where it preferentially corrodes to protect the base metal, makes it highly effective for rust prevention. However, its reactivity with acids renders it less corrosion-resistant than chromium or nickel in certain environments. Zinc plating is the most cost-effective option among the three.
In terms of cost hierarchy, chromium plating is the most expensive, followed by nickel, and then zinc. The plating method also influences cost: hanging plating, which offers better coverage and uniformity, is more expensive than barrel plating, which is more economical for small parts in large quantities.
For quick visual identification:

Extended Knowledge:
The electroplating industry faces significant environmental challenges, particularly regarding heavy metal pollution in wastewater. Consequently, many governments have implemented strict controls on the expansion of electroplating operations, with a trend towards industry reduction and consolidation.
In China, the primary electroplating processes are zinc (50% of total production), copper, nickel, and chromium (each accounting for about 30% of the remaining production). This distribution reflects both market demand and environmental considerations.
For specific applications:
The selection of plating type should consider factors such as the base material, environmental exposure, functional requirements, cost constraints, and regulatory compliance.







