Have you ever wondered why stainless steel, known for its rust resistance, sometimes still shows signs of rust? This article explores the science behind stainless steel’s composition and the factors affecting its durability. You’ll learn practical tips to prevent rust and maintain its shine.

Stainless Steel Definition
Stainless steel is a versatile alloy primarily composed of iron, with a minimum of 10.5% chromium (Cr) and typically less than 1.2% carbon (C). This precise combination of elements creates a protective chromium oxide layer on the surface, giving stainless steel its characteristic corrosion resistance.
In the manufacturing process of stainless steel, various alloying elements are strategically added to enhance specific properties and performance characteristics. These elements include:
The careful balance of these elements results in a material that is not only resistant to rust and corrosion but also exhibits excellent mechanical properties, making it suitable for a wide range of applications across industries. This unique combination of properties is what truly makes the steel “stainless” and highly valued in modern engineering and manufacturing.

The production of stainless steel is a sophisticated metallurgical process that involves the precise addition of various alloying elements in specific quantities to achieve desired performance characteristics. This careful manipulation of composition results in a wide spectrum of stainless steel grades, each tailored for specific applications and environments.
Stainless steel grades are categorized based on their chemical composition, microstructure, and resulting properties. The primary alloying elements, such as chromium, nickel, molybdenum, and nitrogen, are adjusted to enhance corrosion resistance, mechanical strength, ductility, and other crucial attributes.
For instance, austenitic stainless steels (300 series) typically contain higher levels of nickel for improved formability and corrosion resistance, while ferritic grades (400 series) rely more on chromium for their properties. Martensitic stainless steels incorporate carbon for increased hardness and strength, making them suitable for cutting tools and surgical instruments.
The table below outlines common alloying elements used in decorative stainless steel, their typical composition ranges, and primary effects on the material’s properties. It is important to note that this information is provided for reference purposes only, and specific grade selection should involve consultation with metallurgical experts or material specifications.
Stainless Steel Chemical Composition Chart (percentage %)
| Steel Grades | C | Si | Mn | P | S | Cr | Ni |
|---|---|---|---|---|---|---|---|
| 304 | ≤0.08 | ≤1.00 | ≤2.00 | ≤0.045 | ≤0.03 | 18-20 | 8-10 |
| 301 | ≤0.15 | ≤1.00 | ≤2.00 | ≤0.045 | ≤0.03 | 16-18 | 6-8 |
| 202 | ≤0.15 | ≤1.00 | 7.5-10 | ≤0.05 | ≤0.03 | 17-19 | 4-6 |
| 201 | ≤0.15 | ≤1.00 | 5.5-7.5 | ≤0.05 | ≤0.03 | 16-18 | 3.5-5.5 |
Understanding these grades allows engineers, designers, and manufacturers to select the most appropriate stainless steel for their specific requirements, balancing factors such as corrosion resistance, mechanical properties, formability, and cost-effectiveness.

Alloy Element Composition
The corrosion resistance of stainless steel is primarily determined by its chromium content. A minimum of 10.5% chromium is required to form a passive chromium oxide film, which provides basic corrosion protection.
Higher chromium and nickel contents generally enhance corrosion resistance. For instance, 304 stainless steel, containing 18-20% chromium and 8-10% nickel, exhibits excellent corrosion resistance in many environments.
Other alloying elements such as molybdenum, nitrogen, and titanium can further improve specific corrosion-resistant properties. For example, 316 stainless steel, with 2-3% molybdenum addition, offers superior resistance to pitting and crevice corrosion in chloride-rich environments.
Manufacturing Process Quality
The metallurgical quality of stainless steel significantly impacts its corrosion resistance. Advanced manufacturing processes ensure:
Large, technologically advanced steel mills typically achieve superior quality control, resulting in more consistent and reliable corrosion-resistant products. Conversely, facilities with suboptimal equipment or processes may produce stainless steel with compromised corrosion resistance due to compositional inconsistencies, residual impurities, or inadequate microstructural control.
Environmental Exposure
The service environment plays a crucial role in stainless steel’s corrosion behavior:
Proper material selection, considering both alloy composition and environmental factors, is essential for ensuring optimal corrosion resistance in specific applications.
The common practice of using a magnet to test stainless steel quality is based on a widespread misconception. While many consumers believe non-magnetic stainless steel is inherently rust-resistant, the magnetic properties of stainless steel are actually determined by its microstructure rather than its corrosion resistance.
Stainless steel’s microstructure is primarily influenced by its chemical composition and the thermal processing it undergoes during manufacturing. The three main microstructures in stainless steel are ferrite, austenite, and martensite. Ferritic and martensitic stainless steels are magnetic, while austenitic stainless steel is generally non-magnetic. However, it’s crucial to note that austenitic stainless steel, despite its excellent mechanical properties and weldability, may not always outperform ferritic stainless steel in terms of corrosion resistance.
The market offers various grades of stainless steel, including those in the 200 and 300 series with low nickel and high manganese content. These grades are non-magnetic but often exhibit inferior performance characteristics compared to the industry-standard 304 stainless steel, which contains a higher nickel content. It’s worth noting that even 304 stainless steel can display slight magnetic properties after certain manufacturing processes such as cold drawing, stress-relief annealing, precision polishing, or investment casting.
Furthermore, the corrosion resistance of stainless steel is primarily attributed to its chromium content, which forms a protective passive layer on the surface. This passive layer is present in both magnetic and non-magnetic stainless steels, making the magnetic test an unreliable indicator of corrosion resistance.
In conclusion, using magnetic properties as a sole criterion for judging stainless steel quality is scientifically unsound and can lead to erroneous conclusions about material performance. A comprehensive evaluation considering factors such as chemical composition, intended application, and specific grade properties is essential for selecting the appropriate stainless steel for a given purpose.
Many consumers carry a small magnet when shopping for stainless steel, believing that non-magnetic stainless steel is inherently rust-proof. However, this is a misconception. The magnetic properties of stainless steel are determined by its crystalline structure, not its corrosion resistance.
Customers are often surprised to see brown rust spots on stainless steel surfaces, assuming that “stainless” means it never rusts, and if it does, the material must be defective. This view, however, oversimplifies the complex nature of stainless steel.
In reality, stainless steel can corrode under specific conditions. While it resists atmospheric oxidation, its resistance to corrosion in acidic, alkaline, and saline environments varies based on several factors:
For instance, 304 stainless steel exhibits excellent corrosion resistance in dry, clean atmospheres. However, when exposed to coastal environments, it can rapidly corrode due to chloride-rich sea mist. This demonstrates that not all stainless steel grades can resist corrosion universally.
The corrosion resistance of stainless steel stems from a thin, continuous, and stable chromium-rich oxide film on its surface. This passive layer prevents oxygen infiltration and subsequent oxidation of the underlying metal. However, if this protective film is continuously compromised, atmospheric oxygen and moisture can penetrate and react with iron atoms, leading to progressive surface rusting.
Several mechanisms can damage the protective film on stainless steel surfaces:
The conditions mentioned above can compromise the integrity of the passive chromium oxide film on the stainless steel surface, leading to localized corrosion and potential rusting.
To maintain a pristine, corrosion-resistant metal surface, we recommend implementing the following best practices:
By adhering to these guidelines, you can significantly extend the service life of stainless steel components and maintain their aesthetic appeal in various environmental conditions.
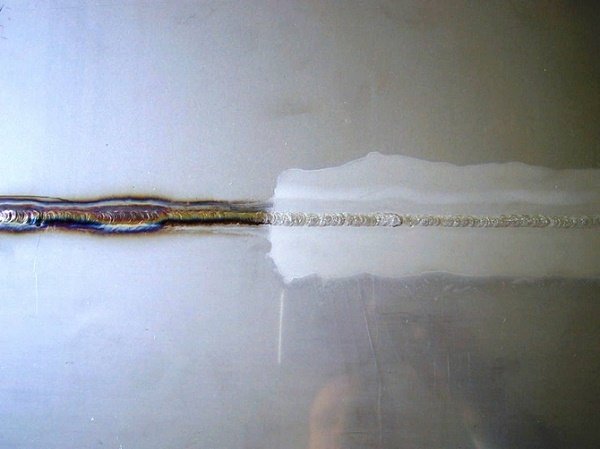
Applying pickling paste or spray effectively removes rust and regenerates the protective chromium oxide film, restoring the stainless steel’s corrosion resistance. This process involves:
Post-treatment, employ fine-grit polishing equipment to restore the surface finish, followed by applying a high-quality polishing wax to seal and protect the surface.
For minor rust stains, a 1:1 mixture of mineral spirits and light machine oil can be effective. Apply the solution, allow it to penetrate, then wipe clean with a microfiber cloth.
b) Mechanical Methods:

Mechanical rust removal techniques include:
These methods effectively remove surface contaminants, including embedded iron particles, which are particularly problematic in humid environments. However, it’s crucial to perform mechanical cleaning when the surface is dry to prevent moisture-induced flash rusting.
Note that while mechanical cleaning effectively removes surface rust, it doesn’t inherently improve the material’s corrosion resistance. To enhance protection:
By combining appropriate chemical and mechanical methods, followed by proper finishing techniques, the corrosion resistance and aesthetic appeal of stainless steel can be effectively restored and maintained.